mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern
- PMID: 35090580
- PMCID: PMC8733218
- DOI: 10.1016/j.immuni.2022.01.001
mRNA-1273 vaccine-induced antibodies maintain Fc effector functions across SARS-CoV-2 variants of concern
Abstract
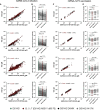
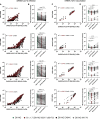
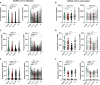
SARS-CoV-2 mRNA vaccines confer robust protection against COVID-19, but the emergence of variants has generated concerns regarding the protective efficacy of the currently approved vaccines, which lose neutralizing potency against some variants. Emerging data suggest that antibody functions beyond neutralization may contribute to protection from the disease, but little is known about SARS-CoV-2 antibody effector functions. Here, we profiled the binding and functional capacity of convalescent antibodies and Moderna mRNA-1273 COVID-19 vaccine-induced antibodies across SARS-CoV-2 variants of concern (VOCs). Although the neutralizing responses to VOCs decreased in both groups, the Fc-mediated responses were distinct. In convalescent individuals, although antibodies exhibited robust binding to VOCs, they showed compromised interactions with Fc-receptors. Conversely, vaccine-induced antibodies also bound robustly to VOCs but continued to interact with Fc-receptors and mediate antibody effector functions. These data point to a resilience in the mRNA-vaccine-induced humoral immune response that may continue to offer protection from SARS-CoV-2 VOCs independent of neutralization.
Keywords: COVID-19; Fc effector function; SARS-CoV-2; mRNA-1273 vaccination; vaccines; variants of concern.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.A. is the founder of Seromyx Systems Inc. A.C. is an employee of Moderna Inc. D.D., P.M., A.S.M., and E.R.M. are employees of Space Exploration Technologies Corp. All other authors have declared no competing interests.
Figures

Similar articles
-
SARS-CoV-2 BA.4/5 infection triggers more cross-reactive FcγRIIIa signaling and neutralization than BA.1, in the context of hybrid immunity.J Virol. 2024 Jul 23;98(7):e0067824. doi: 10.1128/jvi.00678-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953380 Free PMC article.
-
SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees.Sci Immunol. 2021 May 25;6(59):eabj1750. doi: 10.1126/sciimmunol.abj1750. Sci Immunol. 2021. PMID: 34035118 Free PMC article.
-
Safety and immunogenicity of a modified mRNA-lipid nanoparticle vaccine candidate against COVID-19: Results from a phase 1, dose-escalation study.Hum Vaccin Immunother. 2024 Dec 31;20(1):2408863. doi: 10.1080/21645515.2024.2408863. Epub 2024 Oct 18. Hum Vaccin Immunother. 2024. PMID: 39422261 Free PMC article. Clinical Trial.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
The Key to Increase Immunogenicity of Next-Generation COVID-19 Vaccines Lies in the Inclusion of the SARS-CoV-2 Nucleocapsid Protein.J Immunol Res. 2024 May 29;2024:9313267. doi: 10.1155/2024/9313267. eCollection 2024. J Immunol Res. 2024. PMID: 38939745 Free PMC article. Review.
Cited by
-
Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant.bioRxiv [Preprint]. 2022 Sep 13:2022.09.12.507614. doi: 10.1101/2022.09.12.507614. bioRxiv. 2022. Update in: Nat Med. 2023 Jan;29(1):247-257. doi: 10.1038/s41591-022-02092-8 PMID: 36263060 Free PMC article. Updated. Preprint.
-
SARS-CoV-2 BA.4/5 infection triggers more cross-reactive FcγRIIIa signaling and neutralization than BA.1, in the context of hybrid immunity.J Virol. 2024 Jul 23;98(7):e0067824. doi: 10.1128/jvi.00678-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953380 Free PMC article.
-
Antibody-dependent cellular cytotoxicity against SARS-CoV-2 Omicron sub-lineages is reduced in convalescent sera regardless of infecting variant.Cell Rep Med. 2023 Jan 17;4(1):100910. doi: 10.1016/j.xcrm.2022.100910. Epub 2022 Dec 22. Cell Rep Med. 2023. PMID: 36603577 Free PMC article.
-
SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals.Cell Host Microbe. 2022 Jun 8;30(6):880-886.e4. doi: 10.1016/j.chom.2022.03.029. Epub 2022 Mar 25. Cell Host Microbe. 2022. PMID: 35436444 Free PMC article.
-
Molecular characteristics, immune evasion, and impact of SARS-CoV-2 variants.Signal Transduct Target Ther. 2022 Jun 28;7(1):202. doi: 10.1038/s41392-022-01039-2. Signal Transduct Target Ther. 2022. PMID: 35764603 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- U19 AI135995/AI/NIAID NIH HHS/United States
- R01 AI042790/AI/NIAID NIH HHS/United States
- R01 AI146785/AI/NIAID NIH HHS/United States
- UM1 AI148373/AI/NIAID NIH HHS/United States
- HHSN272201500002C/AI/NIAID NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- UM1 AI148576/AI/NIAID NIH HHS/United States
- U01 CA260476/CA/NCI NIH HHS/United States
- R37 AI080289/AI/NIAID NIH HHS/United States
- U01 AI149644/AI/NIAID NIH HHS/United States
- UM1 AI148684/AI/NIAID NIH HHS/United States
- 75N93019C00052/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

