Intranasal COVID-19 vaccines: From bench to bed
- PMID: 35085851
- PMCID: PMC8785603
- DOI: 10.1016/j.ebiom.2022.103841
Intranasal COVID-19 vaccines: From bench to bed
Abstract
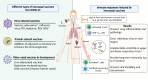
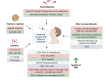
Currently licensed COVID-19 vaccines are all designed for intramuscular (IM) immunization. However, vaccination today failed to prevent the virus infection through the upper respiratory tract, which is partially due to the absence of mucosal immunity activation. Despite the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants, the next generation of COVID-19 vaccine is in demand and intranasal (IN) vaccination method has been demonstrated to be potent in inducing both mucosal and systemic immune responses. Presently, although not licensed, various IN vaccines against SARS-CoV-2 are under intensive investigation, with 12 candidates reaching clinical trials at different phases. In this review, we give a detailed description about current status of IN COVID-19 vaccines, including virus-vectored vaccines, recombinant subunit vaccines and live attenuated vaccines. The ongoing clinical trials for IN vaccines are highlighted. Additionally, the underlying mechanisms of mucosal immunity and potential mucosal adjuvants and nasal delivery devices are also summarized.
Keywords: Adjuvant; COVID-19; Intranasal vaccine; Mucosal; SARS-CoV-2.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no conflicts of interests.
Figures

Similar articles
-
Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2.Cell. 2022 Mar 3;185(5):896-915.e19. doi: 10.1016/j.cell.2022.02.005. Epub 2022 Feb 9. Cell. 2022. PMID: 35180381 Free PMC article.
-
Nasal prevention of SARS-CoV-2 infection by intranasal influenza-based boost vaccination in mouse models.EBioMedicine. 2022 Jan;75:103762. doi: 10.1016/j.ebiom.2021.103762. Epub 2021 Dec 21. EBioMedicine. 2022. PMID: 34942445 Free PMC article.
-
Toll-like Receptor-4 (TLR4) Agonist-Based Intranasal Nanovaccine Delivery System for Inducing Systemic and Mucosal Immunity.Mol Pharm. 2021 Jun 7;18(6):2233-2241. doi: 10.1021/acs.molpharmaceut.0c01256. Epub 2021 May 19. Mol Pharm. 2021. PMID: 34010002
-
Intranasal vaccines for SARS-CoV-2: From challenges to potential in COVID-19 management.Drug Discov Today. 2021 Nov;26(11):2619-2636. doi: 10.1016/j.drudis.2021.07.021. Epub 2021 Jul 29. Drug Discov Today. 2021. PMID: 34332100 Free PMC article. Review.
-
Prospects for mucosal vaccine: shutting the door on SARS-CoV-2.Hum Vaccin Immunother. 2020 Dec 1;16(12):2921-2931. doi: 10.1080/21645515.2020.1805992. Epub 2020 Sep 15. Hum Vaccin Immunother. 2020. PMID: 32931361 Free PMC article. Review.
Cited by
-
Saliva is suitable for SARS-CoV-2 antibodies detection after vaccination: A rapid systematic review.Front Immunol. 2022 Sep 20;13:1006040. doi: 10.3389/fimmu.2022.1006040. eCollection 2022. Front Immunol. 2022. PMID: 36203571 Free PMC article.
-
Propagation of SARS-CoV-2 in a Closed Cell Culture Device: Potential GMP Compatible Production Platform for Live-Attenuated Vaccine Candidates under BSL-3 Conditions?Viruses. 2023 Jan 30;15(2):397. doi: 10.3390/v15020397. Viruses. 2023. PMID: 36851610 Free PMC article.
-
Understanding the Roles of Host Defense Peptides in Immune Modulation: From Antimicrobial Action to Potential as Adjuvants.J Microbiol Biotechnol. 2023 Mar 28;33(3):288-298. doi: 10.4014/jmb.2301.01005. Epub 2023 Feb 10. J Microbiol Biotechnol. 2023. PMID: 36775853 Free PMC article. Review.
-
Is heterologous prime-boost COVID-19 vaccination a concern or an opportunity for Ethiopia?Front Public Health. 2023 Jan 26;10:1046546. doi: 10.3389/fpubh.2022.1046546. eCollection 2022. Front Public Health. 2023. PMID: 36777764 Free PMC article. No abstract available.
-
Aerosol Inhalation of Chimpanzee Adenovirus Vectors (ChAd68) Expressing Ancestral or Omicron BA.1 Stabilized Pre-Fusion Spike Glycoproteins Protects Non-Human Primates against SARS-CoV-2 Infection.Vaccines (Basel). 2023 Aug 28;11(9):1427. doi: 10.3390/vaccines11091427. Vaccines (Basel). 2023. PMID: 37766104 Free PMC article.
References
-
- WHO’s landscape of COVID-19 vaccine candidates. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19..., 24-November 2021.
-
- Registry data of COVID-19 vaccine candidates. https://covid19.trackvaccines.org/, 24-November 2021.
-
- Kim J.H., Marks F., Clemens J.D. Looking beyond COVID-19 vaccine phase 3 trials. Nat Med. 2021;27(2):205–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

