Acetylated α-tubulin K394 regulates microtubule stability to shape the growth of axon terminals
- PMID: 35081332
- PMCID: PMC8843987
- DOI: 10.1016/j.cub.2021.12.012
Acetylated α-tubulin K394 regulates microtubule stability to shape the growth of axon terminals
Abstract
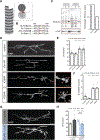
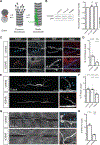
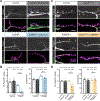
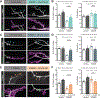
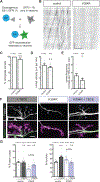
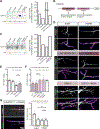
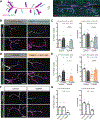
Microtubules are essential to neuron shape and function. Acetylation of tubulin has the potential to directly tune the behavior and function of microtubules in cells. Although proteomic studies have identified several acetylation sites in α-tubulin, the effects of acetylation at these sites remains largely unknown. This includes the highly conserved residue lysine 394 (K394), which is located at the αβ-tubulin dimer interface. Using a fly model, we show that α-tubulin K394 is acetylated in the nervous system and is an essential residue. We found that an acetylation-blocking mutation in endogenous α-tubulin, K394R, perturbs the synaptic morphogenesis of motoneurons and reduces microtubule stability. Intriguingly, the K394R mutation has opposite effects on the growth of two functionally and morphologically distinct motoneurons, revealing neuron-type-specific responses when microtubule stability is altered. Eliminating the deacetylase HDAC6 increases K394 acetylation, and the over-expression of HDAC6 reduces microtubule stability similar to the K394R mutant. Thus, our findings implicate α-tubulin K394 and its acetylation in the regulation of microtubule stability and suggest that HDAC6 regulates K394 acetylation during synaptic morphogenesis.
Keywords: Drosophila; acetylation; cytoskeleton; microtubule; neuron; synaptic morphogenesis.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Comment in
-
Microtubule regulation: Transcending the tenet of K40 acetylation.Curr Biol. 2022 Feb 7;32(3):R126-R128. doi: 10.1016/j.cub.2021.12.018. Curr Biol. 2022. PMID: 35134360
Similar articles
-
Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells.J Biol Chem. 2013 Aug 2;288(31):22516-26. doi: 10.1074/jbc.M113.489328. Epub 2013 Jun 24. J Biol Chem. 2013. PMID: 23798680 Free PMC article.
-
HDAC6 is a microtubule-associated deacetylase.Nature. 2002 May 23;417(6887):455-8. doi: 10.1038/417455a. Nature. 2002. PMID: 12024216
-
MeCP2 deficiency is associated with reduced levels of tubulin acetylation and can be restored using HDAC6 inhibitors.J Mol Med (Berl). 2015 Jan;93(1):63-72. doi: 10.1007/s00109-014-1202-x. Epub 2014 Sep 12. J Mol Med (Berl). 2015. PMID: 25209898
-
Tubulin acetylation: responsible enzymes, biological functions and human diseases.Cell Mol Life Sci. 2015 Nov;72(22):4237-55. doi: 10.1007/s00018-015-2000-5. Epub 2015 Jul 31. Cell Mol Life Sci. 2015. PMID: 26227334 Free PMC article. Review.
-
HDAC6 α-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases.J Neurol Sci. 2011 May 15;304(1-2):1-8. doi: 10.1016/j.jns.2011.02.017. Epub 2011 Mar 5. J Neurol Sci. 2011. PMID: 21377170 Review.
Cited by
-
FTD-associated mutations in Tau result in a combination of dominant and recessive phenotypes.Neurobiol Dis. 2022 Aug;170:105770. doi: 10.1016/j.nbd.2022.105770. Epub 2022 May 16. Neurobiol Dis. 2022. PMID: 35588988 Free PMC article.
-
Interaction Between Aging-Related Elastin-Derived Peptide (VGVAPG) and Sirtuin 2 and its Impact on Functions of Human Neuron Cells in an In Vitro Model.Mol Neurobiol. 2025 Jan;62(1):819-831. doi: 10.1007/s12035-024-04298-y. Epub 2024 Jun 24. Mol Neurobiol. 2025. PMID: 38914873 Free PMC article.
-
Endothelial cell elongation and alignment in response to shear stress requires acetylation of microtubules.Front Physiol. 2024 Sep 10;15:1425620. doi: 10.3389/fphys.2024.1425620. eCollection 2024. Front Physiol. 2024. PMID: 39318362 Free PMC article.
-
α-Tubulin acetylation at lysine 40 regulates dendritic arborization and larval locomotion by promoting microtubule stability in Drosophila.PLoS One. 2023 Feb 24;18(2):e0280573. doi: 10.1371/journal.pone.0280573. eCollection 2023. PLoS One. 2023. PMID: 36827311 Free PMC article.
-
TTC5 syndrome: Clinical and molecular spectrum of a severe and recognizable condition.Am J Med Genet A. 2022 Sep;188(9):2652-2665. doi: 10.1002/ajmg.a.62852. Epub 2022 Jun 7. Am J Med Genet A. 2022. PMID: 35670379 Free PMC article.
References
-
- Penazzi L, Bakota L, and Brandt R (2016). Microtubule Dynamics in Neuronal Development, Plasticity, and Neurodegeneration. International Review of Cell and Molecular Biology 321, 89–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

