Chloride channels with ClC-1-like properties differentially regulate the excitability of dopamine receptor D1- and D2-expressing striatal medium spiny neurons
- PMID: 35080921
- PMCID: PMC8917939
- DOI: 10.1152/ajpcell.00397.2021
Chloride channels with ClC-1-like properties differentially regulate the excitability of dopamine receptor D1- and D2-expressing striatal medium spiny neurons
Abstract
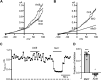
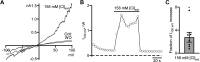
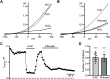
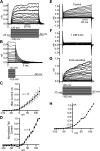
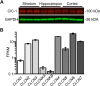
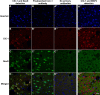
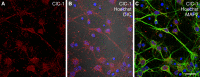
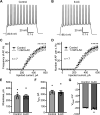
Dynamic chloride (Cl-) regulation is critical for synaptic inhibition. In mature neurons, Cl- influx and extrusion are primarily controlled by ligand-gated anion channels (GABAA and glycine receptors) and the potassium chloride cotransporter K+-Cl- cotransporter 2 (KCC2), respectively. Here, we report for the first time, to our knowledge, a presence of a new source of Cl- influx in striatal neurons with properties similar to chloride voltage-gated channel 1 (ClC-1). Using whole cell patch-clamp recordings, we detected an outwardly rectifying voltage-dependent current that was impermeable to the large anion methanesulfonate (MsO-). The anionic current was sensitive to the ClC-1 inhibitor 9-anthracenecarboxylic acid (9-AC) and the nonspecific blocker phloretin. The mean fractions of anionic current inhibition by MsO-, 9-AC, and phloretin were not significantly different, indicating that anionic current was caused by active ClC-1-like channels. In addition, we found that Cl- current was not sensitive to the transmembrane protein 16A (TMEM16A; Ano1) inhibitor Ani9 and that the outward Cl- rectification was preserved even at a very high intracellular Ca2+ concentration (2 mM), indicating that TMEM16B (Ano2) did not contribute to the total current. Western blotting and immunohistochemical analyses confirmed the presence of ClC-1 channels in the striatum mainly localized to the somata of striatal neurons. Finally, we found that 9-AC decreased action potential firing frequencies and increased excitability in medium spiny neurons (MSNs) expressing dopamine type 1 (D1) and type 2 (D2) receptors in the brain slices, respectively. We conclude that ClC-1-like channels are preferentially located at the somata of MSNs, are functional, and can modulate neuronal excitability.
Keywords: ClC-1, chloride voltage-gated channel 1; TMEM16A, transmembrane protein 16A (Ano1); chloride homeostasis; dopamine type 1 and type 2 receptors; striatal medium spiny neurons.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures
Similar articles
-
Novel voltage-dependent Cl- channels in striatal medium spiny neurons are unrelated to ClC-1 or other known Ca2+-induced Cl- channel/transporter types.Neurosci Lett. 2025 Jan 1;844:138032. doi: 10.1016/j.neulet.2024.138032. Epub 2024 Nov 2. Neurosci Lett. 2025. PMID: 39491780
-
Differential dopaminergic regulation of inwardly rectifying potassium channel mediated subthreshold dynamics in striatal medium spiny neurons.Neuropharmacology. 2016 Aug;107:396-410. doi: 10.1016/j.neuropharm.2016.03.037. Epub 2016 Mar 24. Neuropharmacology. 2016. PMID: 27018450
-
Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons.Eur J Neurosci. 2008 Feb;27(3):671-82. doi: 10.1111/j.1460-9568.2008.06038.x. Eur J Neurosci. 2008. PMID: 18279319
-
D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons.Trends Neurosci. 2007 May;30(5):228-35. doi: 10.1016/j.tins.2007.03.008. Epub 2007 Apr 3. Trends Neurosci. 2007. PMID: 17408758 Review.
-
The physiological roles of anoctamin2/TMEM16B and anoctamin1/TMEM16A in chemical senses.Cell Calcium. 2024 Jun;120:102889. doi: 10.1016/j.ceca.2024.102889. Epub 2024 Apr 18. Cell Calcium. 2024. PMID: 38677213 Review.
Cited by
-
Sustained fentanyl exposure inhibits neuronal activity in dissociated striatal neuronal-glial cocultures through actions independent of opioid receptors.J Neurophysiol. 2024 Sep 1;132(3):1056-1073. doi: 10.1152/jn.00444.2023. Epub 2024 Aug 7. J Neurophysiol. 2024. PMID: 39110896
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

