Extracellular Vesicles of Mesenchymal Stromal Cells Can be Taken Up by Microglial Cells and Partially Prevent the Stimulation Induced by β-amyloid
- PMID: 35080744
- PMCID: PMC8942956
- DOI: 10.1007/s12015-021-10261-4
Extracellular Vesicles of Mesenchymal Stromal Cells Can be Taken Up by Microglial Cells and Partially Prevent the Stimulation Induced by β-amyloid
Abstract
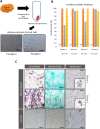
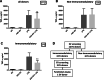
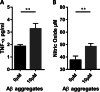
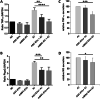
Mesenchymal stromal/stem cells (MSCs) have great capacity for immune regulation. MSCs provide protective paracrine effects, which are partially exerted by extracellular vesicles (EVs). It has been reported that MSCs-derived EVs (MSC-EVs) contain soluble factors, such as cytokines, chemokines, growth factors and even microRNAs, which confer them similar anti-inflammatory and regenerative effects to MSCs. Moreover, MSCs modulate microglia activation through a dual mechanism of action that relies both on cell contact and secreted factors. Microglia cells are the central nervous system immune cells and the main mediators of the inflammation leading to neurodegenerative disorders. Here, we investigated whether MSC-EVs affect the activation of microglia cells by β-amyloid aggregates. We show that the presence of MSC-EVs can prevent the upregulation of pro-inflammatory mediators such as tumor necrosis factor (TNF)-α and nitric oxide (NO). Both are up-regulated in neurodegenerative diseases representing chronic inflammation, as in Alzheimer's disease. We demonstrate that MSC-EVs are internalized by the microglia cells. Further, our study supports the use of MSC-EVs as a promising therapeutic tool to treat neuroinflammatory diseases.Significance StatementIt has been reported that mesenchymal stromal/stem cells and MSC-derived small extracellular vesicles have therapeutic effects in the treatment of various degenerative and inflammatory diseases. Extracellular vesicles are loaded with proteins, lipids and RNA and act as intercellular communication mediators. Here we show that extracellular vesicles can be taken up by murine microglial cells. In addition, they partially reduce the activation of microglial cells against β-amyloid aggregates. This inhibition of microglia activation may present an effective strategy for the control/therapy of neurodegenerative diseases such as Alzheimer's disease.
Keywords: Alzheimer Disease; Amyloid beta; Extracellular vesicles; Mesenchymal stromal/stem cells; Microglia; Neuroinflammation.
© 2021. The Author(s).
Conflict of interest statement
We have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Mesenchymal stem cell-derived extracellular vesicles carrying miR-99b-3p restrain microglial activation and neuropathic pain by stimulating autophagy.Int Immunopharmacol. 2023 Feb;115:109695. doi: 10.1016/j.intimp.2023.109695. Epub 2023 Jan 11. Int Immunopharmacol. 2023. PMID: 36638658
-
Mesenchymal stem cells-derived extracellular vesicles ameliorate Alzheimer's disease in rat models via the microRNA-29c-3p/BACE1 axis and the Wnt/β-catenin pathway.Aging (Albany NY). 2021 Jun 4;13(11):15285-15306. doi: 10.18632/aging.203088. Epub 2021 Jun 4. Aging (Albany NY). 2021. PMID: 34086603 Free PMC article.
-
Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease.Stem Cells Transl Med. 2020 Sep;9(9):1068-1084. doi: 10.1002/sctm.19-0327. Epub 2020 Jun 4. Stem Cells Transl Med. 2020. PMID: 32496649 Free PMC article.
-
Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis.Cell Tissue Res. 2018 Oct;374(1):1-15. doi: 10.1007/s00441-018-2871-5. Epub 2018 Jun 28. Cell Tissue Res. 2018. PMID: 29955951 Review.
-
The immunomodulatory effects of mesenchymal stem cell-derived extracellular vesicles in Alzheimer's disease.Front Immunol. 2024 Jan 8;14:1325530. doi: 10.3389/fimmu.2023.1325530. eCollection 2023. Front Immunol. 2024. PMID: 38259476 Free PMC article. Review.
Cited by
-
Extracellular vesicle therapy in neurological disorders.J Biomed Sci. 2024 Aug 25;31(1):85. doi: 10.1186/s12929-024-01075-w. J Biomed Sci. 2024. PMID: 39183263 Free PMC article. Review.
-
High throughput screening of mesenchymal stromal cell morphological response to inflammatory signals for bioreactor-based manufacturing of extracellular vesicles that modulate microglia.bioRxiv [Preprint]. 2023 Nov 19:2023.11.19.567730. doi: 10.1101/2023.11.19.567730. bioRxiv. 2023. Update in: Bioact Mater. 2024 Mar 20;37:153-171. doi: 10.1016/j.bioactmat.2024.03.009 PMID: 38014258 Free PMC article. Updated. Preprint.
-
Current Advances and Future Perspectives on Mesenchymal Stem Cell-Derived Extracellular Vesicles in Alzheimer's Disease.Aging Dis. 2024 Oct 1;15(5):2015-2027. doi: 10.14336/AD.2023.1206. Aging Dis. 2024. PMID: 38270122 Free PMC article. Review.
-
Therapeutic efficacy and promise of stem cell-derived extracellular vesicles in Alzheimer's disease and other aging-related disorders.Ageing Res Rev. 2023 Dec;92:102088. doi: 10.1016/j.arr.2023.102088. Epub 2023 Oct 11. Ageing Res Rev. 2023. PMID: 37827304 Free PMC article. Review.
-
High throughput screening of mesenchymal stromal cell morphological response to inflammatory signals for bioreactor-based manufacturing of extracellular vesicles that modulate microglia.Bioact Mater. 2024 Mar 20;37:153-171. doi: 10.1016/j.bioactmat.2024.03.009. eCollection 2024 Jul. Bioact Mater. 2024. PMID: 38549769 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

