High-resolution structures of a thermophilic eukaryotic 80S ribosome reveal atomistic details of translocation
- PMID: 35079002
- PMCID: PMC8789840
- DOI: 10.1038/s41467-022-27967-9
High-resolution structures of a thermophilic eukaryotic 80S ribosome reveal atomistic details of translocation
Abstract
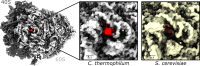
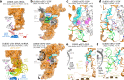
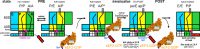
Ribosomes are complex and highly conserved ribonucleoprotein assemblies catalyzing protein biosynthesis in every organism. Here we present high-resolution cryo-EM structures of the 80S ribosome from a thermophilic fungus in two rotational states, which due to increased 80S stability provide a number of mechanistic details of eukaryotic translation. We identify a universally conserved 'nested base-triple knot' in the 26S rRNA at the polypeptide tunnel exit with a bulged-out nucleotide that likely serves as an adaptable element for nascent chain containment and handover. We visualize the structure and dynamics of the ribosome protective factor Stm1 upon ribosomal 40S head swiveling. We describe the structural impact of a unique and essential m1acp3 Ψ 18S rRNA hyper-modification embracing the anticodon wobble-position for eukaryotic tRNA and mRNA translocation. We complete the eEF2-GTPase switch cycle describing the GDP-bound post-hydrolysis state. Taken together, our data and their integration into the structural landscape of 80S ribosomes furthers our understanding of protein biogenesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



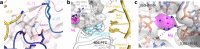
Similar articles
-
Structures of modified eEF2 80S ribosome complexes reveal the role of GTP hydrolysis in translocation.EMBO J. 2007 May 2;26(9):2421-31. doi: 10.1038/sj.emboj.7601677. Epub 2007 Apr 19. EMBO J. 2007. PMID: 17446867 Free PMC article.
-
Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation.EMBO J. 2004 Mar 10;23(5):1008-19. doi: 10.1038/sj.emboj.7600102. Epub 2004 Feb 19. EMBO J. 2004. PMID: 14976550 Free PMC article.
-
Structural Insights into the Role of Diphthamide on Elongation Factor 2 in mRNA Reading-Frame Maintenance.J Mol Biol. 2018 Aug 17;430(17):2677-2687. doi: 10.1016/j.jmb.2018.06.006. Epub 2018 Jun 7. J Mol Biol. 2018. PMID: 29886014
-
Three-dimensional electron cryomicroscopy of ribosomes.Curr Protein Pept Sci. 2002 Feb;3(1):79-91. doi: 10.2174/1389203023380873. Curr Protein Pept Sci. 2002. PMID: 12370013 Review.
-
Atomic structures of the eukaryotic ribosome.Trends Biochem Sci. 2012 May;37(5):189-98. doi: 10.1016/j.tibs.2012.02.007. Epub 2012 Mar 20. Trends Biochem Sci. 2012. PMID: 22436288 Review.
Cited by
-
Structural inventory of cotranslational protein folding by the eukaryotic RAC complex.Nat Struct Mol Biol. 2023 May;30(5):670-677. doi: 10.1038/s41594-023-00973-1. Epub 2023 Apr 20. Nat Struct Mol Biol. 2023. PMID: 37081320 Free PMC article.
-
Protein translation: biological processes and therapeutic strategies for human diseases.Signal Transduct Target Ther. 2024 Feb 23;9(1):44. doi: 10.1038/s41392-024-01749-9. Signal Transduct Target Ther. 2024. PMID: 38388452 Free PMC article. Review.
-
Beyond the Backbone: The Next Generation of Pathwalking Utilities for Model Building in CryoEM Density Maps.Biomolecules. 2022 Jun 2;12(6):773. doi: 10.3390/biom12060773. Biomolecules. 2022. PMID: 35740898 Free PMC article.
-
Methionine aminopeptidase 2 and its autoproteolysis product have different binding sites on the ribosome.Nat Commun. 2024 Jan 24;15(1):716. doi: 10.1038/s41467-024-44862-7. Nat Commun. 2024. PMID: 38267453 Free PMC article.
-
A Homologous Recombination System to Generate Epitope-Tagged Target Genes in Chaetomium thermophilum: A Genetic Approach to Investigate Native Thermostable Proteins.Int J Mol Sci. 2022 Mar 16;23(6):3198. doi: 10.3390/ijms23063198. Int J Mol Sci. 2022. PMID: 35328616 Free PMC article.
References
-
- Ben-Shem A, et al. The structure of the eukaryotic ribosome at 3.0 A resolution. Science. 2011;334:1524–1529. - PubMed
-
- Anger AM, et al. Structures of the human and Drosophila 80S ribosome. Nature. 2013;497:80–85. - PubMed
-
- Khatter H, Myasnikov AG, Natchiar SK, Klaholz BP. Structure of the human 80S ribosome. Nature. 2015;520:640–645. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

