Myo-Inositol in Fermented Sugar Matrix Improves Human Macrophage Function
- PMID: 35073444
- PMCID: PMC9420542
- DOI: 10.1002/mnfr.202100852
Myo-Inositol in Fermented Sugar Matrix Improves Human Macrophage Function
Abstract
Scope: Reactive oxygen species production by innate immune cells plays a central role in host defense against invading pathogens at wound-site. A weakened host-defense results in persistent infection leading to wound chronicity. Fermented Papaya Preparation (FPP), a complex sugar matrix, bolsters respiratory burst activity and improves wound healing outcomes in chronic wound patients. The objective of the current study was to identify underlying molecular factor/s responsible for augmenting macrophage host defense mechanisms following FPP supplementation.
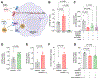
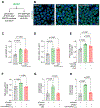
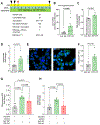
Methods and results: In depth LC-MS/MS analysis of cells supplemented with FPP led to identification of myo-inositol as a key determinant of FPP activity towards improving macrophage function. Myo-inositol, in quantities that is present in FPP, significantly improved macrophage respiratory burst and phagocytosis via de novo synthesis pathway of ISYNA1. In addition, myo-inositol transporters, HMIT and SMIT1, played a significant role in such activity. Blocking these pathways using siRNA attenuated FPP-induced improved macrophage host defense activities. FPP supplementation emerged as a novel approach to increase intracellular myo-inositol levels. Such supplementation also modified wound microenvironment in chronic wound patients to augment myo-inositol levels in wound fluid.
Conclusion: These observations indicate that myo-inositol in FPP influences multiple aspects of macrophage function critical for host defense against invading pathogens.
Keywords: ROS; dietary supplement; macrophage; myo-inositol; phagocytosis.
© 2022 Wiley-VCH GmbH.
Figures

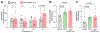

Similar articles
-
May Dietary Supplementation Augment Respiratory Burst in Wound-Site Inflammatory Cells?Antioxid Redox Signal. 2018 Feb 10;28(5):401-405. doi: 10.1089/ars.2017.7304. Epub 2017 Oct 16. Antioxid Redox Signal. 2018. PMID: 28810801 Free PMC article.
-
Improved function of diabetic wound-site macrophages and accelerated wound closure in response to oral supplementation of a fermented papaya preparation.Antioxid Redox Signal. 2010 Sep 1;13(5):599-606. doi: 10.1089/ars.2009.3039. Antioxid Redox Signal. 2010. PMID: 20095880 Free PMC article.
-
Contributions in astrocytes of SMIT1/2 and HMIT to myo-inositol uptake at different concentrations and pH.Neurochem Int. 2012 Jul;61(2):187-94. doi: 10.1016/j.neuint.2012.04.010. Epub 2012 Apr 27. Neurochem Int. 2012. PMID: 22564531
-
Potential role and therapeutic interests of myo-inositol in metabolic diseases.Biochimie. 2013 Oct;95(10):1811-27. doi: 10.1016/j.biochi.2013.05.011. Epub 2013 Jun 10. Biochimie. 2013. PMID: 23764390 Review.
-
Myo-inositol Effects on the Developing Respiratory Neural Control System.Adv Exp Med Biol. 2018;1071:159-166. doi: 10.1007/978-3-319-91137-3_20. Adv Exp Med Biol. 2018. PMID: 30357747 Review.
Cited by
-
Primary Human M2 Macrophage Subtypes Are Distinguishable by Aqueous Metabolite Profiles.Int J Mol Sci. 2024 Feb 18;25(4):2407. doi: 10.3390/ijms25042407. Int J Mol Sci. 2024. PMID: 38397084 Free PMC article.
References
-
- Altay F, Karbancioglu-Guler F, Daskaya-Dikmen C, Heperkan D, A review on traditional Turkish fermented non-alcoholic beverages: microbiota, fermentation process and quality characteristics. Int J Food Microbiol 2013, 167, 44–56. - PubMed
-
- Sanlier N, Gokcen BB, Sezgin AC, Health benefits of fermented foods. Crit Rev Food Sci Nutr 2019, 59, 506–527. - PubMed
-
- Shiferaw Terefe N, in: Smithers G (Ed.), Elsevier Food Science Reference module, Elsevier; 2015, pp. 1–3.
-
- Hwang J, Kim JC, Moon H, Yang JY, Kim M, Determination of sodium contents in traditional fermented foods in Korea. J Food Compos Anal 2017, 56, 110–114.
-
- Rubio CA, Schmidt PT, Severe Defects in the Macrophage Barrier to Gut Microflora in Inflammatory Bowel Disease and Colon Cancer. Anticancer Res 2018, 38, 3811–3815. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

