Immune function and dysfunction are determined by lymphoid tissue efficacy
- PMID: 35072206
- PMCID: PMC8807573
- DOI: 10.1242/dmm.049256
Immune function and dysfunction are determined by lymphoid tissue efficacy
Abstract
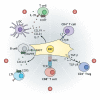
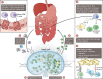
Lymphoid tissue returns to a steady state once each immune response is resolved, and although this occurs multiple times throughout life, its structural integrity and functionality remain unaffected. Stromal cells orchestrate cellular interactions within lymphoid tissue, and any changes to the microenvironment can have detrimental outcomes and drive disease. A breakdown in lymphoid tissue homeostasis can lead to a loss of tissue structure and function that can cause aberrant immune responses. This Review highlights recent advances in our understanding of lymphoid tissue function and remodelling in adaptive immunity and in disease states. We discuss the functional role of lymphoid tissue in disease progression and explore the changes to lymphoid tissue structure and function driven by infection, chronic inflammatory conditions and cancer. Understanding the role of lymphoid tissues in immune responses to a wide range of pathologies allows us to take a fuller systemic view of disease progression.
Keywords: Fibroblastic reticular cells; Homeostasis; Lymphoid tissue; Stromal cells.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

Similar articles
-
Fibroblastic reticular cells at the nexus of innate and adaptive immune responses.Immunol Rev. 2019 May;289(1):31-41. doi: 10.1111/imr.12748. Immunol Rev. 2019. PMID: 30977192 Free PMC article. Review.
-
Stromal cell networks coordinate immune response generation and maintenance.Immunol Rev. 2018 May;283(1):77-85. doi: 10.1111/imr.12641. Immunol Rev. 2018. PMID: 29664562 Review.
-
Stromal cell regulation of homeostatic and inflammatory lymphoid organogenesis.Immunology. 2013 Sep;140(1):12-21. doi: 10.1111/imm.12119. Immunology. 2013. PMID: 23621403 Free PMC article. Review.
-
Aging of lymphoid stromal architecture impacts immune responses.Semin Immunol. 2023 Nov;70:101817. doi: 10.1016/j.smim.2023.101817. Epub 2023 Aug 10. Semin Immunol. 2023. PMID: 37572552 Free PMC article. Review.
-
Role of adipose-associated lymphoid tissues in the immunological homeostasis of the serosal surface.Immunol Lett. 2020 Dec;228:135-141. doi: 10.1016/j.imlet.2020.11.001. Epub 2020 Nov 6. Immunol Lett. 2020. PMID: 33166529 Review.
Cited by
-
Application of hydrogels in cancer immunotherapy: a bibliometric analysis.Front Immunol. 2024 Aug 13;15:1433050. doi: 10.3389/fimmu.2024.1433050. eCollection 2024. Front Immunol. 2024. PMID: 39192983 Free PMC article.
-
Anti-PD-L1 Immunotherapy of Chronic Virus Infection Improves Virus Control without Augmenting Tissue Damage by Fibrosis.Viruses. 2024 May 17;16(5):799. doi: 10.3390/v16050799. Viruses. 2024. PMID: 38793680 Free PMC article.
-
Bottom-up synthetic immunology.Nat Nanotechnol. 2024 Nov;19(11):1587-1596. doi: 10.1038/s41565-024-01744-9. Epub 2024 Aug 26. Nat Nanotechnol. 2024. PMID: 39187581 Review.
-
Effects of fine particulate matter on bone marrow-conserved hematopoietic and mesenchymal stem cells: a systematic review.Exp Mol Med. 2024 Feb;56(1):118-128. doi: 10.1038/s12276-023-01149-z. Epub 2024 Jan 10. Exp Mol Med. 2024. PMID: 38200155 Free PMC article. Review.
-
Differential kinetics of splenic CD169+ macrophage death is one underlying cause of virus infection fate regulation.Cell Death Dis. 2023 Dec 18;14(12):838. doi: 10.1038/s41419-023-06374-y. Cell Death Dis. 2023. PMID: 38110339 Free PMC article.
References
-
- Acton, S. E., Astarita, J. L., Malhotra, D., Lukacs-Kornek, V., Franz, B., Hess, P. R., Jakus, Z., Kuligowski, M., Fletcher, A. L., Elpek, K. G.et al. (2012). Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 37, 276-289. 10.1016/j.immuni.2012.05.022 - DOI - PMC - PubMed
-
- Acton, S. E., Farrugia, A. J., Astarita, J. L., Mourão-Sá, D., Jenkins, R. P., Nye, E., Hooper, S., van Blijswijk, J., Rogers, N. C., Snelgrove, K. J.et al. (2014). Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature 514, 498-502. 10.1038/nature13814 - DOI - PMC - PubMed
-
- Albrengues, J., Bertero, T., Grasset, E., Bonan, S., Maiel, M., Bourget, I., Philippe, C., Serrano, C. H., Benamar, S., Croce, O.et al. (2015). Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat. Commun. 6, 10204-10215. 10.1038/ncomms10204 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

