Cholinergic Signaling Attenuates Pro-Inflammatory Interleukin-8 Response in Colonic Epithelial Cells
- PMID: 35069554
- PMCID: PMC8770536
- DOI: 10.3389/fimmu.2021.781147
Cholinergic Signaling Attenuates Pro-Inflammatory Interleukin-8 Response in Colonic Epithelial Cells
Abstract
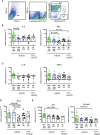
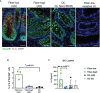
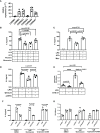
Infants affected by Hirschsprung disease (HSCR), a neurodevelopmental congenital disorder, lack ganglia of the intrinsic enteric nervous system (aganglionosis) in a variable length of the colon, and are prone to developing severe Hirschsprung-associated enterocolitis (HAEC). HSCR patients typically show abnormal dense innervation of extrinsic cholinergic nerve fibers throughout the aganglionic rectosigmoid. Cholinergic signaling has been reported to reduce inflammatory response. Consequently, a sparse extrinsic cholinergic innervation in the mucosa of the rectosigmoid correlates with increased inflammatory immune cell frequencies and higher incidence of HAEC in HSCR patients. However, whether cholinergic signals influence the pro-inflammatory immune response of intestinal epithelial cells (IEC) is unknown. Here, we analyzed colonic IEC isolated from 43 HSCR patients with either a low or high mucosal cholinergic innervation density (fiber-low versus fiber-high) as well as from control tissue. Compared to fiber-high samples, IEC purified from fiber-low rectosigmoid expressed significantly higher levels of IL-8 but not TNF-α, IL-10, TGF-β1, Muc-2 or tight junction proteins. IEC from fiber-low rectosigmoid showed higher IL-8 protein concentrations in cell lysates as well as prominent IL-8 immunoreactivity compared to IEC from fiber-high tissue. Using the human colonic IEC cell line SW480 we demonstrated that cholinergic signals suppress lipopolysaccharide-induced IL-8 secretion via the alpha 7 nicotinic acetylcholine receptor (a7nAChR). In conclusion, we showed for the first time that the presence of a dense mucosal cholinergic innervation is associated with decreased secretion of IEC-derived pro-inflammatory IL-8 in the rectosigmoid of HSCR patients likely dependent on a7nAChR activation. Owing to the association between IL-8 and enterocolitis-prone, fiber-low HSCR patients, targeted therapies against IL-8 might be a promising immunotherapy candidate for HAEC treatment.
Keywords: Hirschsprung disease; Hirschsprung-associated enterocolitis; Interleukin-8; acetylcholine receptors; intestinal epithelial cell.
Copyright © 2022 Müller, Kym, Galati, Tharakan, Subotic, Krebs, Stathopoulos, Schmittenbecher, Cholewa, Romero, Reingruber, NIGStudy Group, Holland-Cunz and Keck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Lack of Mucosal Cholinergic Innervation Is Associated With Increased Risk of Enterocolitis in Hirschsprung's Disease.Cell Mol Gastroenterol Hepatol. 2021;12(2):507-545. doi: 10.1016/j.jcmgh.2021.03.004. Epub 2021 Mar 16. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33741501 Free PMC article.
-
Associations of Mucosal Nerve Fiber Innervation Density with Hirschsprung-Associated Enterocolitis: A Retrospective Three-Center Cohort Study.Eur J Pediatr Surg. 2023 Aug;33(4):299-309. doi: 10.1055/a-1889-6355. Epub 2022 Jul 1. Eur J Pediatr Surg. 2023. PMID: 35777734
-
Gfra1 Underexpression Causes Hirschsprung's Disease and Associated Enterocolitis in Mice.Cell Mol Gastroenterol Hepatol. 2019;7(3):655-678. doi: 10.1016/j.jcmgh.2018.12.007. Epub 2018 Dec 27. Cell Mol Gastroenterol Hepatol. 2019. PMID: 30594740 Free PMC article.
-
Inflammatory bowel disease in patients with Hirschsprung's disease: a systematic review and meta-analysis.Pediatr Surg Int. 2018 Feb;34(2):149-154. doi: 10.1007/s00383-017-4182-4. Epub 2017 Oct 5. Pediatr Surg Int. 2018. PMID: 28983688 Review.
-
Hirschsprung's disease--immunohistochemical findings.Histol Histopathol. 1994 Jul;9(3):615-29. Histol Histopathol. 1994. PMID: 7981507 Review.
Cited by
-
Bioelectric regulation of intestinal stem cells.Trends Cell Biol. 2023 Jul;33(7):555-567. doi: 10.1016/j.tcb.2022.10.003. Epub 2022 Nov 15. Trends Cell Biol. 2023. PMID: 36396487 Free PMC article. Review.
-
Neonatal development of intestinal neuroimmune interactions.Trends Neurosci. 2022 Dec;45(12):928-941. doi: 10.1016/j.tins.2022.10.002. Epub 2022 Oct 28. Trends Neurosci. 2022. PMID: 36404456 Free PMC article. Review.
-
A Novel Method for Identifying the Transition Zone in Long-Segment Hirschsprung Disease: Investigating the Muscle Unit to Ganglion Ratio.Biomolecules. 2022 Aug 10;12(8):1101. doi: 10.3390/biom12081101. Biomolecules. 2022. PMID: 36008996 Free PMC article.
-
Neuroimmune regulation in Hirschsprung's disease associated enterocolitis.Front Immunol. 2023 Apr 17;14:1127375. doi: 10.3389/fimmu.2023.1127375. eCollection 2023. Front Immunol. 2023. PMID: 37138874 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

