Coordinated Translocation of Presequence-Containing Precursor Proteins Across Two Mitochondrial Membranes: Knowns and Unknowns of How TOM and TIM23 Complexes Cooperate With Each Other
- PMID: 35069261
- PMCID: PMC8770809
- DOI: 10.3389/fphys.2021.806426
Coordinated Translocation of Presequence-Containing Precursor Proteins Across Two Mitochondrial Membranes: Knowns and Unknowns of How TOM and TIM23 Complexes Cooperate With Each Other
Abstract
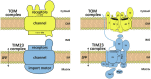
The vast majority of mitochondrial proteins are encoded in the nuclear genome and synthesized on cytosolic ribosomes as precursor proteins with specific mitochondrial targeting signals. Mitochondrial targeting signals are very diverse, however, about 70% of mitochondrial proteins carry cleavable, N-terminal extensions called presequences. These amphipathic helices with one positively charged and one hydrophobic surface target proteins to the mitochondrial matrix with the help of the TOM and TIM23 complexes in the outer and inner membranes, respectively. Translocation of proteins across the two mitochondrial membranes does not take place independently of each other. Rather, in the intermembrane space, where the two complexes meet, components of the TOM and TIM23 complexes form an intricate network of protein-protein interactions that mediates initially transfer of presequences and then of the entire precursor proteins from the outer to the inner mitochondrial membrane. In this Mini Review, we summarize our current understanding of how the TOM and TIM23 complexes cooperate with each other and highlight some of the future challenges and unresolved questions in the field.
Keywords: TIM23 complex; TOM complex; TOM-TIM23 contacts; intermembrane space; mitochondria; precursor transfer; presequence pathway; protein translocation.
Copyright © 2022 Genge and Mokranjac.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
How to get to the other side of the mitochondrial inner membrane - the protein import motor.Biol Chem. 2020 May 26;401(6-7):723-736. doi: 10.1515/hsz-2020-0106. Biol Chem. 2020. PMID: 32142474 Review.
-
From TOM to the TIM23 complex - handing over of a precursor.Biol Chem. 2020 May 26;401(6-7):709-721. doi: 10.1515/hsz-2020-0101. Biol Chem. 2020. PMID: 32074073 Review.
-
TOM-TIM23 supercomplex formation.Methods Enzymol. 2024;707:3-22. doi: 10.1016/bs.mie.2024.07.042. Epub 2024 Aug 24. Methods Enzymol. 2024. PMID: 39488380
-
On the mechanism of preprotein import by the mitochondrial presequence translocase.Biochim Biophys Acta. 2010 Jun;1803(6):732-9. doi: 10.1016/j.bbamcr.2010.01.013. Epub 2010 Jan 25. Biochim Biophys Acta. 2010. PMID: 20100523 Review.
-
Role of Tim50 in the transfer of precursor proteins from the outer to the inner membrane of mitochondria.Mol Biol Cell. 2009 Mar;20(5):1400-7. doi: 10.1091/mbc.e08-09-0934. Epub 2009 Jan 14. Mol Biol Cell. 2009. PMID: 19144822 Free PMC article.
Cited by
-
Role of UPRmt and mitochondrial dynamics in host immunity: it takes two to tango.Front Cell Infect Microbiol. 2023 May 16;13:1135203. doi: 10.3389/fcimb.2023.1135203. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37260703 Free PMC article. Review.
-
Interactions of amyloidogenic proteins with mitochondrial protein import machinery in aging-related neurodegenerative diseases.Front Physiol. 2023 Nov 2;14:1263420. doi: 10.3389/fphys.2023.1263420. eCollection 2023. Front Physiol. 2023. PMID: 38028797 Free PMC article. Review.
-
NLG1, encoding a mitochondrial membrane protein, controls leaf and grain development in rice.BMC Plant Biol. 2023 Sep 9;23(1):418. doi: 10.1186/s12870-023-04417-2. BMC Plant Biol. 2023. PMID: 37689677 Free PMC article.
-
Two domains of Tim50 coordinate translocation of proteins across the two mitochondrial membranes.Life Sci Alliance. 2023 Sep 25;6(12):e202302122. doi: 10.26508/lsa.202302122. Print 2023 Dec. Life Sci Alliance. 2023. PMID: 37748811 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

