Salvianolic acid B alleviates diabetic endothelial and mitochondrial dysfunction by down-regulating apoptosis and mitophagy of endothelial cells
- PMID: 35068334
- PMCID: PMC8974099
- DOI: 10.1080/21655979.2022.2026552
Salvianolic acid B alleviates diabetic endothelial and mitochondrial dysfunction by down-regulating apoptosis and mitophagy of endothelial cells
Abstract
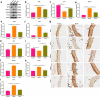
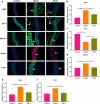
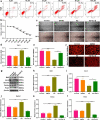
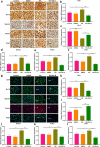
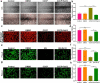
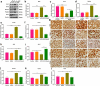
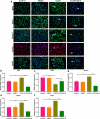
Endothelial dysfunction is a critical mediator in the pathogenesis of vascular complications of diabetes. Herein, this study was conducted to investigate the therapeutic effects of Salvianolic acid B (Sal B) on diabetes-induced endothelial dysfunction and the underlying mechanisms. Diabetic models were established both in db/db mice and high glucose (HG)-induced human umbilical vein endothelial cells (HUVECs). Moreover, HUVECs were exposed to carbonyl cyanide m-chlorophenyl hydrazone (CCCP) to induce endothelial cell damage. Following Sal B treatment, pathological changes of thoracic aorta were investigated by hematoxylin and eosin, alcian blue (AB), elastic fiber, Masson, and reticular fiber staining. BCL2-associated X (BAX), B-cell lymphoma-2 (Bcl-2), Beclin1, Parkin and PTEN Induced Kinase 1 (Pink1) expression was detected by Western blot, immunohistochemistry, and immunofluorescence in thoracic aorta, HG- and CCCP-induced HUVECs. Cell scratch test, MitoTracker Red CMXRos staining and Flou-4 AM staining were separately presented to detect migration, mitochondrial activity and intracellular Ca2+ in HUVECs. Our results showed that Sal B significantly ameliorated hyperlipidemia, hyperglycemia, hyperinsulinemia, and insulin resistance in db/db mice. Furthermore, it significantly alleviated diabetes-induced vascular endothelial dysfunction according to histopathology analysis. In diabetic thoracic aorta, HG- and CCCP-induced HUVECs, Sal B distinctly increased Bcl-2 expression and reduced BAX, Beclin1, Parkin and Pink1 expression, thereby protecting endothelial cells from apoptosis and mitophagy. Moreover, Sal B markedly enhanced migration, mitochondrial activity and intracellular Ca2+ levels both in HG- and CCCP-induced HUVECs. Collectively, Sal B exhibited a potential to improve diabetes-induced endothelial and mitochondrial dysfunction through down-regulating apoptosis and mitophagy of endothelial cells.Abbreviations: DM: diabetes mellitus; T2DM: type 2 diabetes mellitus; Sal B: Salvianolic acid B; HG: high glucose; FBG: fasting blood glucose; TC: total cholesterol; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; FINS: fasting insulin; HOMA-IR: homeostasis model assessment insulin resistance; QUICKI: quantitative insulin-sensitivity check index; H&E: hematoxylin and eosin; HUVECs: human umbilical vein endothelial cells; IHC: immunohistochemistry; CCCP: carbonyl cyanide m-chlorophenyl hydrazone; FCM: flow cytometry; CCK-8: cell counting kit-8.
Keywords: Salvianolic acid B; apoptosis; diabetes; endothelial dysfunction; mitophagy.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

Similar articles
-
Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/Parkin-mediated mitophagy.J Ethnopharmacol. 2021 May 10;271:113855. doi: 10.1016/j.jep.2021.113855. Epub 2021 Jan 22. J Ethnopharmacol. 2021. PMID: 33485979
-
Salvianolic acid B protects against oxLDL-induced endothelial dysfunction under high-glucose conditions by downregulating ROCK1-mediated mitophagy and apoptosis.Biochem Pharmacol. 2020 Apr;174:113815. doi: 10.1016/j.bcp.2020.113815. Epub 2020 Jan 20. Biochem Pharmacol. 2020. PMID: 31972167
-
Salvianolic acid B improves vascular endothelial function in diabetic rats with blood glucose fluctuations via suppression of endothelial cell apoptosis.Eur J Pharmacol. 2016 Nov 15;791:308-315. doi: 10.1016/j.ejphar.2016.09.014. Epub 2016 Sep 8. Eur J Pharmacol. 2016. PMID: 27614127
-
Mitochondrial regulation of diabetic vascular disease: an emerging opportunity.Transl Res. 2018 Dec;202:83-98. doi: 10.1016/j.trsl.2018.07.015. Epub 2018 Aug 4. Transl Res. 2018. PMID: 30144425 Free PMC article. Review.
-
Molecular mechanisms of coronary microvascular endothelial dysfunction in diabetes mellitus: focus on mitochondrial quality surveillance.Angiogenesis. 2022 Aug;25(3):307-329. doi: 10.1007/s10456-022-09835-8. Epub 2022 Mar 18. Angiogenesis. 2022. PMID: 35303170 Review.
Cited by
-
Mitophagy in atherosclerosis: from mechanism to therapy.Front Immunol. 2023 May 16;14:1165507. doi: 10.3389/fimmu.2023.1165507. eCollection 2023. Front Immunol. 2023. PMID: 37261351 Free PMC article. Review.
-
Endothelium-dependent vasorelaxant effects of praeruptorin a in isolated rat thoracic aorta.Bioengineered. 2022 Apr;13(4):10038-10046. doi: 10.1080/21655979.2022.2062979. Bioengineered. 2022. PMID: 35416124 Free PMC article.
-
Exploring the Mechanism of Salvianolic Acid B against Myocardial Ischemia-Reperfusion Injury Based on Network Pharmacology.Pharmaceuticals (Basel). 2024 Feb 28;17(3):309. doi: 10.3390/ph17030309. Pharmaceuticals (Basel). 2024. PMID: 38543095 Free PMC article.
-
Molecular Mechanisms of Plant Extracts in Protecting Aging Blood Vessels.Nutrients. 2024 Jul 20;16(14):2357. doi: 10.3390/nu16142357. Nutrients. 2024. PMID: 39064801 Free PMC article. Review.
-
Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle?Medicina (Kaunas). 2022 Mar 25;58(4):472. doi: 10.3390/medicina58040472. Medicina (Kaunas). 2022. PMID: 35454311 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
