Neonatal rotavirus vaccine (RV3-BB) immunogenicity and safety in a neonatal and infant administration schedule in Malawi: a randomised, double-blind, four-arm parallel group dose-ranging study
- PMID: 35065683
- PMCID: PMC9021029
- DOI: 10.1016/S1473-3099(21)00473-4
Neonatal rotavirus vaccine (RV3-BB) immunogenicity and safety in a neonatal and infant administration schedule in Malawi: a randomised, double-blind, four-arm parallel group dose-ranging study
Abstract
Background: Rotavirus vaccines reduce rotavirus-related deaths and hospitalisations but are less effective in high child mortality countries. The human RV3-BB neonatal G3P[6] rotavirus vaccine administered in a neonatal schedule was efficacious in reducing severe rotavirus gastroenteritis in Indonesia but had not yet been evaluated in African infants.
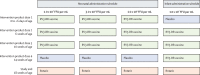
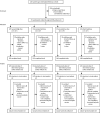
Methods: We did a phase 2, randomised, double-blind, parallel group dose-ranging study of three doses of oral RV3-BB rotavirus vaccine in infants in three primary health centres in Blantyre, Malawi. Healthy infants less than 6 days of age with a birthweight 2·5 to 4·0 kg were randomly assigned (1:1:1:1) into one of four treatment groups: neonatal vaccine group, which included high-titre (1·0 × 107 focus-forming unit [FFU] per mL), mid-titre (3·0 × 106 FFU per mL), or low-titre (1·0 × 106 FFU per mL); and infant vaccine group, which included high-titre (1·0 × 107 FFU per mL) using a computer generated code (block size of four), stratified by birth (singleton vs multiple). Neonates received their three doses at 0-5 days to 10 weeks and infants at 6-14 weeks. Investigators, participant families, and laboratory staff were masked to group allocation. Anti-rotavirus IgA seroconversion and vaccine take (IgA seroconversion and stool shedding) were evaluated. Safety was assessed in all participants who received at least one dose of vaccine or placebo. The primary outcome was the cumulative IgA seroconversion 4 weeks after three doses of RV3-BB in the neonatal schedule in the high-titre, mid-titre, and low-titre groups in the per protocol population, with its 95% CI. With the high-titre group as the active control group, we did a non-inferiority analysis of the proportion of participants with IgA seroconversion in the mid-titre and low-titre groups, using a non-inferiority margin of less than 20%. This trial is registered at ClinicalTrials.gov (NCT03483116).
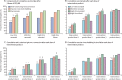
Findings: Between Sept 17, 2018, and Jan 27, 2020, 711 participants recruited were randomly assigned into four treatment groups (neonatal schedule high titre n=178, mid titre n=179, low titre n=175, or infant schedule high titre n=179). In the neonatal schedule, cumulative IgA seroconversion 4 weeks after three doses of RV3-BB was observed in 79 (57%) of 139 participants in the high-titre group, 80 (57%) of 141 participants in the mid-titre group, and 57 (41%) of 138 participants in the low-titre group and at 18 weeks in 100 (72%) of 139 participants in the high-titre group, 96 (67%) of 143 participants in the mid-titre group, and 86 (62%) of 138 of participants in the low-titre. No difference in cumulative IgA seroconversion 4 weeks after three doses of RV3-BB was observed between high-titre and mid-titre groups in the neonatal schedule (difference in response rate 0·001 [95%CI -0·115 to 0·117]), fulfilling the criteria for non-inferiority. In the infant schedule group 82 (59%) of 139 participants had a cumulative IgA seroconversion 4 weeks after three doses of RV3-BB at 18 weeks. Cumulative vaccine take was detected in 483 (85%) of 565 participants at 18 weeks. Three doses of RV3-BB were well tolerated with no difference in adverse events among treatment groups: 67 (39%) of 170 participants had at least one adverse event in the high titre group, 68 (40%) of 172 participants had at least one adverse event in the mid titre group, and 69 (41%) of 169 participants had at least one adverse event in the low titre group.
Interpretation: RV3-BB was well tolerated and immunogenic when co-administered with Expanded Programme on Immunisation vaccines in a neonatal or infant schedule. A lower titre (mid-titre) vaccine generated similar IgA seroconversion to the high-titre vaccine presenting an opportunity to enhance manufacturing capacity and reduce costs. Neonatal administration of the RV3-BB vaccine has the potential to improve protection against rotavirus disease in children in a high-child mortality country in Africa.
Funding: Bill & Melinda Gates Foundation, Australian Tropical Medicine Commercialisation Grant.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests JEB is the lead of the Enteric Diseases group at MCRI (MCRI holds the license for RV3-BB vaccine). JEB is the Director of the Australian Rotavirus Surveillance Program funded by the Australian Government Department of Health and GlaxoSmithKline. MCRI and GLB hold the patent for the RV3-BB vaccine. NAC is affiliated to the National for Health Research Health Protection Research Unit in Gastrointestinal Infections at University of Liverpool, in partnership with Public Health England, in collaboration with University of Warwick. NAC is based at The University of Liverpool. The views expressed are those of the authors and not necessarily those of the National Institute of Health Research, the Department of Health and Social Care, or Public Health England. CMD has served on advisory boards for GSK (between 2019 and 2021) with all payments directed to an administrative fund held by MCRI.
Figures
Similar articles
-
Leveraging Beneficial Off-Target Effects of Live-Attenuated Rotavirus Vaccines.Vaccines (Basel). 2022 Mar 10;10(3):418. doi: 10.3390/vaccines10030418. Vaccines (Basel). 2022. PMID: 35335050 Free PMC article. Review.
-
Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial.Lancet Infect Dis. 2015 Dec;15(12):1389-97. doi: 10.1016/S1473-3099(15)00227-3. Epub 2015 Aug 26. Lancet Infect Dis. 2015. PMID: 26318715 Clinical Trial.
-
Human Neonatal Rotavirus Vaccine (RV3-BB) to Target Rotavirus from Birth.N Engl J Med. 2018 Feb 22;378(8):719-730. doi: 10.1056/NEJMoa1706804. N Engl J Med. 2018. PMID: 29466164 Free PMC article. Clinical Trial.
-
Phase I trial of RV3-BB rotavirus vaccine: a human neonatal rotavirus vaccine.Vaccine. 2013 May 28;31(23):2610-6. doi: 10.1016/j.vaccine.2013.04.008. Epub 2013 Apr 16. Vaccine. 2013. PMID: 23597719 Clinical Trial.
-
Early exposure of infants to natural rotavirus infection: a review of studies with human rotavirus vaccine RIX4414.BMC Pediatr. 2014 Nov 30;14:295. doi: 10.1186/s12887-014-0295-2. BMC Pediatr. 2014. PMID: 25433534 Free PMC article. Review.
Cited by
-
Correlates of immune protection against human rotaviruses: natural infection and vaccination.Arch Virol. 2024 Mar 8;169(3):72. doi: 10.1007/s00705-024-05975-y. Arch Virol. 2024. PMID: 38459213 Review.
-
Current and next-generation formulation strategies for inactivated polio vaccines to lower costs, increase coverage, and facilitate polio eradication.Hum Vaccin Immunother. 2022 Dec 30;18(7):2154100. doi: 10.1080/21645515.2022.2154100. Epub 2022 Dec 28. Hum Vaccin Immunother. 2022. PMID: 36576132 Free PMC article. Review.
-
Immunogenicity and safety of a new hexavalent rotavirus vaccine in Chinese infants: A randomized, double-blind, placebo-controlled phase 2 clinical trial.Hum Vaccin Immunother. 2023 Aug;19(2):2263228. doi: 10.1080/21645515.2023.2263228. Epub 2023 Oct 16. Hum Vaccin Immunother. 2023. PMID: 37843437 Free PMC article. Clinical Trial.
-
The Frequency of Rotavirus Gastroenteritis in Children from West of Iran and Genotyping of Rotavirus Isolates: A Suggestion for Further Changes in Childhood Immunization Program.J Res Health Sci. 2024 Aug 1;24(3):e00621. doi: 10.34172/jrhs.2024.156. Epub 2024 Jul 31. J Res Health Sci. 2024. PMID: 39311104 Free PMC article.
-
Leveraging Beneficial Off-Target Effects of Live-Attenuated Rotavirus Vaccines.Vaccines (Basel). 2022 Mar 10;10(3):418. doi: 10.3390/vaccines10030418. Vaccines (Basel). 2022. PMID: 35335050 Free PMC article. Review.
References
-
- International Vaccine Access Centre Vaccine Information Management System (VIMS) global rotavirus vaccine access report. 2021. https://www.jhsph.edu/research/centers-and-institutes/ivac/view-hub/]
-
- Cameron DJ, Bishop RF, Veenstra AA, Barnes GL, Holmes IH, Ruck BJ. Pattern of shedding of two noncultivable viruses in stools of newborn babies. J Med Virol. 1978;2:7–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

