Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China
- PMID: 35062694
- PMCID: PMC8780113
- DOI: 10.3390/vaccines10010033
Safety and Immunogenicity of a Shigella Bivalent Conjugate Vaccine (ZF0901) in 3-Month- to 5-Year-Old Children in China
Abstract
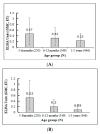
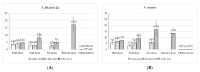
No licensed Shigella vaccine is presently available globally. A double-blinded, randomized, placebo-controlled, age descending phase II clinical trial of a bivalent conjugate vaccine was studied in China. The vaccine ZF0901 consisted of O-specific polysaccharides purified and detoxified from lipopolysaccharide (LPS) of S. flexneri 2a and S. sonnei and covalently bonded to tetanus toxoid. A total of 224, 310, and 434 children, consented by parents or guardians, aged 3 to 6 and 6 to 12 months and 1 to 5 years old, respectively, were injected with half or full doses, with or without adjuvant or control Hib vaccine. There were no serious adverse reactions in all recipients of ZF0901 vaccine independent of age, dosage, number of injections, or the adjuvant status. Thirty days after the last injection, ZF0901 induced robust immune responses with significantly higher levels of type-specific serum antibodies (geometric mean concentrations (GMCs) of IgG anti-LPS) against both serotypes in all age groups compared with the pre-immune or the Hib control (p < 0.0001). Here, we demonstrated that ZF0901 bivalent Shigella conjugate vaccine is safe and immunogenic in infants and young children and is likely suitable for routine immunization.
Keywords: Shigella conjugate vaccine; bivalent; clinical trial; infants and young children; safety and immunogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPAsucc conjugate vaccines in one- to four-year-old children.Pediatr Infect Dis J. 2003 Aug;22(8):701-6. doi: 10.1097/01.inf.0000078156.03697.a5. Pediatr Infect Dis J. 2003. PMID: 12913770 Clinical Trial.
-
Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children.Vaccine. 2010 Mar 2;28(10):2231-2235. doi: 10.1016/j.vaccine.2009.12.050. Epub 2010 Jan 5. Vaccine. 2010. PMID: 20056180 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: a phase 1, dose-escalating, single-blind, randomised, placebo-controlled study.Lancet Infect Dis. 2021 Apr;21(4):546-558. doi: 10.1016/S1473-3099(20)30488-6. Epub 2020 Nov 10. Lancet Infect Dis. 2021. PMID: 33186516 Clinical Trial.
-
Detoxified O-Specific Polysaccharide (O-SP)-Protein Conjugates: Emerging Approach in the Shigella Vaccine Development Scene.Vaccines (Basel). 2022 Apr 24;10(5):675. doi: 10.3390/vaccines10050675. Vaccines (Basel). 2022. PMID: 35632431 Free PMC article. Review.
-
Meningococcal groups C and Y and haemophilus B tetanus toxoid conjugate vaccine (HibMenCY-TT; MenHibrix(®)): a review.Drugs. 2013 May;73(7):703-13. doi: 10.1007/s40265-013-0048-9. Drugs. 2013. PMID: 23649970 Review.
Cited by
-
The Ongoing Journey of a Shigella Bioconjugate Vaccine.Vaccines (Basel). 2022 Jan 29;10(2):212. doi: 10.3390/vaccines10020212. Vaccines (Basel). 2022. PMID: 35214671 Free PMC article. Review.
-
Development of a Shigella conjugate vaccine targeting Shigella flexneri 6 that is immunogenic and provides protection against virulent challenge.Vaccine. 2024 Oct 24;42(24):126263. doi: 10.1016/j.vaccine.2024.126263. Epub 2024 Aug 31. Vaccine. 2024. PMID: 39217775
-
Development of Shigella conjugate vaccines targeting Shigella flexneri 2a and S. flexneri 3a using a simple platform-approach conjugation by squaric acid chemistry.Vaccine. 2023 Jul 31;41(34):4967-4977. doi: 10.1016/j.vaccine.2023.06.052. Epub 2023 Jul 1. Vaccine. 2023. PMID: 37400283 Free PMC article.
-
Shigella Vaccines: The Continuing Unmet Challenge.Int J Mol Sci. 2024 Apr 13;25(8):4329. doi: 10.3390/ijms25084329. Int J Mol Sci. 2024. PMID: 38673913 Free PMC article. Review.
-
Frontiers in Shigella Vaccine Development.Vaccines (Basel). 2022 Sep 15;10(9):1536. doi: 10.3390/vaccines10091536. Vaccines (Basel). 2022. PMID: 36146614 Free PMC article.
References
-
- Khalil I.A., Troeger C., Blacker B.F., Rao P.C., Brown A., Atherly D.E., Brewer T.G., Engmann C.M., Houpt E.R., Kang G., et al. Morbidity and mortality due to Shigella and Enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018;18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

