Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants
- PMID: 35060999
- PMCID: PMC8848203
- DOI: 10.1001/jama.2022.0470
Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants
Abstract
Importance: Assessing COVID-19 vaccine performance against the rapidly spreading SARS-CoV-2 Omicron variant is critical to inform public health guidance.
Objective: To estimate the association between receipt of 3 doses of Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 vaccine and symptomatic SARS-CoV-2 infection, stratified by variant (Omicron and Delta).
Design, setting, and participants: A test-negative case-control analysis among adults 18 years or older with COVID-like illness tested December 10, 2021, through January 1, 2022, by a national pharmacy-based testing program (4666 COVID-19 testing sites across 49 US states).
Exposures: Three doses of mRNA COVID-19 vaccine (third dose ≥14 days before test and ≥6 months after second dose) vs unvaccinated and vs 2 doses 6 months or more before test (ie, eligible for a booster dose).
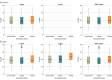
Main outcomes and measures: Association between symptomatic SARS-CoV-2 infection (stratified by Omicron or Delta variants defined using S-gene target failure) and vaccination (3 doses vs unvaccinated and 3 doses vs 2 doses). Associations were measured with multivariable multinomial regression. Among cases, a secondary outcome was median cycle threshold values (inversely proportional to the amount of target nucleic acid present) for 3 viral genes, stratified by variant and vaccination status.
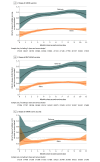
Results: Overall, 23 391 cases (13 098 Omicron; 10 293 Delta) and 46 764 controls were included (mean age, 40.3 [SD, 15.6] years; 42 050 [60.1%] women). Prior receipt of 3 mRNA vaccine doses was reported for 18.6% (n = 2441) of Omicron cases, 6.6% (n = 679) of Delta cases, and 39.7% (n = 18 587) of controls; prior receipt of 2 mRNA vaccine doses was reported for 55.3% (n = 7245), 44.4% (n = 4570), and 41.6% (n = 19 456), respectively; and being unvaccinated was reported for 26.0% (n = 3412), 49.0% (n = 5044), and 18.6% (n = 8721), respectively. The adjusted odds ratio for 3 doses vs unvaccinated was 0.33 (95% CI, 0.31-0.35) for Omicron and 0.065 (95% CI, 0.059-0.071) for Delta; for 3 vaccine doses vs 2 doses the adjusted odds ratio was 0.34 (95% CI, 0.32-0.36) for Omicron and 0.16 (95% CI, 0.14-0.17) for Delta. Median cycle threshold values were significantly higher in cases with 3 doses vs 2 doses for both Omicron and Delta (Omicron N gene: 19.35 vs 18.52; Omicron ORF1ab gene: 19.25 vs 18.40; Delta N gene: 19.07 vs 17.52; Delta ORF1ab gene: 18.70 vs 17.28; Delta S gene: 23.62 vs 20.24).
Conclusions and relevance: Among individuals seeking testing for COVID-like illness in the US in December 2021, receipt of 3 doses of mRNA COVID-19 vaccine (compared with unvaccinated and with receipt of 2 doses) was less likely among cases with symptomatic SARS-CoV-2 infection compared with test-negative controls. These findings suggest that receipt of 3 doses of mRNA vaccine, relative to being unvaccinated and to receipt of 2 doses, was associated with protection against both the Omicron and Delta variants, although the higher odds ratios for Omicron suggest less protection for Omicron than for Delta.
Conflict of interest statement
Figures

Comment in
-
Booster Vaccination to Prevent COVID-19 in the Era of Omicron: An Effective Part of a Layered Public Health Approach.JAMA. 2022 Feb 15;327(7):628-629. doi: 10.1001/jama.2022.0892. JAMA. 2022. PMID: 35061011 No abstract available.
Similar articles
-
COVID-19 Vaccine Effectiveness of Booster Doses Against Delta and Omicron Variants Over Follow-up Times Using Longitudinal Meta-analysis.J Res Health Sci. 2024 Sep 30;24(4):e00626. doi: 10.34172/jrhs.2024.161. Epub 2024 Sep 30. J Res Health Sci. 2024. PMID: 39431651 Free PMC article. Review.
-
Association of Prior BNT162b2 COVID-19 Vaccination With Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance.JAMA. 2022 Jun 14;327(22):2210-2219. doi: 10.1001/jama.2022.7493. JAMA. 2022. PMID: 35560036 Free PMC article.
-
Effect of mRNA Vaccine Boosters against SARS-CoV-2 Omicron Infection in Qatar.N Engl J Med. 2022 May 12;386(19):1804-1816. doi: 10.1056/NEJMoa2200797. Epub 2022 Mar 9. N Engl J Med. 2022. PMID: 35263534 Free PMC article.
-
Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August, 2021-March, 2022: a national, observational, test-negative, case-control study.Lancet Infect Dis. 2023 Apr;23(4):435-444. doi: 10.1016/S1473-3099(22)00729-0. Epub 2022 Nov 24. Lancet Infect Dis. 2023. PMID: 36436536 Free PMC article.
-
The Omicron variant wave: Where are we now and what are the prospects?J Chin Med Assoc. 2023 Feb 1;86(2):135-137. doi: 10.1097/JCMA.0000000000000863. Epub 2022 Dec 13. J Chin Med Assoc. 2023. PMID: 36524941 Review.
Cited by
-
A Population-Based Study of SARS-CoV-2 IgG Antibody Responses to Vaccination in Manitoba.Vaccines (Basel). 2024 Sep 26;12(10):1095. doi: 10.3390/vaccines12101095. Vaccines (Basel). 2024. PMID: 39460263 Free PMC article.
-
COVID-19 Vaccine Effectiveness of Booster Doses Against Delta and Omicron Variants Over Follow-up Times Using Longitudinal Meta-analysis.J Res Health Sci. 2024 Sep 30;24(4):e00626. doi: 10.34172/jrhs.2024.161. Epub 2024 Sep 30. J Res Health Sci. 2024. PMID: 39431651 Free PMC article. Review.
-
Vaccination against rapidly evolving pathogens and the entanglements of memory.Nat Immunol. 2024 Nov;25(11):2015-2023. doi: 10.1038/s41590-024-01970-2. Epub 2024 Oct 9. Nat Immunol. 2024. PMID: 39384979 Review.
-
COVID-19 risk factors and outcomes in individuals with stiff person syndrome spectrum disorders before and after omicron.Orphanet J Rare Dis. 2024 Sep 27;19(1):356. doi: 10.1186/s13023-024-03357-w. Orphanet J Rare Dis. 2024. PMID: 39334235 Free PMC article.
-
Comparative 60-day effectiveness of bivalent versus monovalent mRNA vaccines in Shelby County: a population-level analysis.Ther Adv Vaccines Immunother. 2024 Sep 19;12:25151355241278852. doi: 10.1177/25151355241278852. eCollection 2024. Ther Adv Vaccines Immunother. 2024. PMID: 39314902 Free PMC article.
References
-
- World Health Organization . Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. Updated November 26, 2021. Accessed December 23, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1....
-
- World Health Organization . Enhancing response to OMICRON (COVID-19 variant B.1.1.529): Technical brief and priority actions for Member States. January 7, 2022. Accessed January 11, 2022. https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-...
-
- Centers for Disease Control and Prevention . COVID Data Tracker. Accessed January 7, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

