UL11 Protein Is a Key Participant of the Duck Plague Virus in Its Life Cycle
- PMID: 35058907
- PMCID: PMC8764364
- DOI: 10.3389/fmicb.2021.792361
UL11 Protein Is a Key Participant of the Duck Plague Virus in Its Life Cycle
Abstract
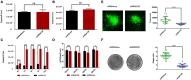
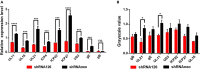
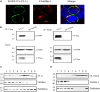
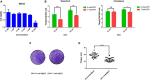
Tegument protein UL11 plays a critical role in the life cycle of herpesviruses. The UL11 protein of herpesviruses is important for viral particle entry, release, assembly, and secondary envelopment. Lipid raft is cholesterol-rich functional microdomains in cell membranes, which plays an important role in signal transduction and substance transport. Flotillin and prohibition, which are considered to be specific markers of lipid raft. However, little is known about the function of duck plague virus (DPV) UL11 in the life cycle of the viruses and the relationship between the lipid raft and UL11. In this study, an interference plasmid shRNA126 for UL11 was used. Results showed that UL11 is involved in the replication, cell to cell spread, viral particle assembly, and release processes. Furthermore, UL11 was verified that it could interact with the lipid raft through sucrose density gradient centrifugation and that function correlates with the second glycine of the UL11. When the lipid raft was depleted using the methyl-β-cyclodextrin, the release of the DPV was decreased. Moreover, UL11 can decrease several relative viral genes mRNA levels by qRT-PCR and Western blot test. Altogether, these results highlight an important role for UL11 protein in the viral replication cycle.
Keywords: Duck plague virus; UL11; lipid raft; prohibition; replication.
Copyright © 2022 Yang, Wang, Cheng, Yang, Wu, Huang, Tian, Jia, Liu, Zhu, Chen, Zhao, Zhang, Ou, Mao, Gao, Sun, Yu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Features and Functions of the Conserved Herpesvirus Tegument Protein UL11 and Its Binding Partners.Front Microbiol. 2022 Jun 3;13:829754. doi: 10.3389/fmicb.2022.829754. eCollection 2022. Front Microbiol. 2022. PMID: 35722336 Free PMC article. Review.
-
The intracellular domain of duck plague virus glycoprotein E affects UL11 protein incorporation into viral particles.Vet Microbiol. 2021 Jun;257:109078. doi: 10.1016/j.vetmic.2021.109078. Epub 2021 Apr 20. Vet Microbiol. 2021. PMID: 33906107
-
Herpes simplex virus protein UL11 but not UL51 is associated with lipid rafts.Virus Genes. 2007 Dec;35(3):571-5. doi: 10.1007/s11262-007-0156-2. Epub 2007 Aug 13. Virus Genes. 2007. PMID: 17694428
-
Myristylation and palmitylation of HSV-1 UL11 are not essential for its function.Virology. 2010 Feb 5;397(1):80-8. doi: 10.1016/j.virol.2009.10.046. Epub 2009 Nov 26. Virology. 2010. PMID: 19944438 Free PMC article.
-
Role of lipids in virus replication.Cold Spring Harb Perspect Biol. 2011 Oct 1;3(10):a004820. doi: 10.1101/cshperspect.a004820. Cold Spring Harb Perspect Biol. 2011. PMID: 21628428 Free PMC article. Review.
Cited by
-
Characterization of a Unique Novel LORF3 Protein of Duck Plague Virus and Its Potential Pathogenesis.J Virol. 2023 Jan 31;97(1):e0157722. doi: 10.1128/jvi.01577-22. Epub 2023 Jan 4. J Virol. 2023. PMID: 36598202 Free PMC article.
-
Regulation of alphaherpesvirus protein via post-translational phosphorylation.Vet Res. 2022 Nov 17;53(1):93. doi: 10.1186/s13567-022-01115-z. Vet Res. 2022. PMID: 36397147 Free PMC article. Review.
-
Alphaherpesvirus glycoprotein E: A review of its interactions with other proteins of the virus and its application in vaccinology.Front Microbiol. 2022 Aug 4;13:970545. doi: 10.3389/fmicb.2022.970545. eCollection 2022. Front Microbiol. 2022. PMID: 35992696 Free PMC article. Review.
-
Epstein-Barr Virus BBLF1 Mediates Secretory Vesicle Transport to Facilitate Mature Virion Release.J Virol. 2023 Jun 29;97(6):e0043723. doi: 10.1128/jvi.00437-23. Epub 2023 May 17. J Virol. 2023. PMID: 37195206 Free PMC article.
-
Features and Functions of the Conserved Herpesvirus Tegument Protein UL11 and Its Binding Partners.Front Microbiol. 2022 Jun 3;13:829754. doi: 10.3389/fmicb.2022.829754. eCollection 2022. Front Microbiol. 2022. PMID: 35722336 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

