A New Series of Indeno[1,2- c]pyrazoles as EGFR TK Inhibitors for NSCLC Therapy
- PMID: 35056800
- PMCID: PMC8778314
- DOI: 10.3390/molecules27020485
A New Series of Indeno[1,2- c]pyrazoles as EGFR TK Inhibitors for NSCLC Therapy
Abstract
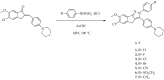
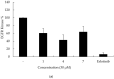
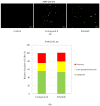
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death throughout the world. Due to the shortcomings of traditional chemotherapy, targeted therapies have come into prominence for the management of NSCLC. In particular, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) therapy has emerged as a first-line therapy for NSCLC patients with EGFR-activating mutations. In this context, new indenopyrazoles, which were prepared by an efficient microwave-assisted method, were subjected to in silico and in vitro assays to evaluate their potency as EGFR TK-targeted anti-NSCLC agents. Compound 4 was the most promising antitumor agent towards A549 human lung adenocarcinoma cells, with an IC50 value of 6.13 µM compared to erlotinib (IC50 = 19.67 µM). Based on its low cytotoxicity to peripheral blood mononuclear cells (PBMCs), it can be concluded that compound 4 exerts selective antitumor action. This compound also inhibited EGFR TK with an IC50 value of 17.58 µM compared to erlotinib (IC50 = 0.04 µM) and induced apoptosis (56.30%). Taking into account in silico and in vitro data, compound 4 stands out as a potential EGFR TKI for the treatment of NSCLC.
Keywords: anticancer activity; apoptosis; epidermal growth factor receptor; indenopyrazoles; microwave-assisted synthesis; non-small cell lung cancer; tyrosine kinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

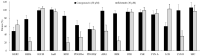

Similar articles
-
Inhibition of histone deacetylases sensitizes EGF receptor-TK inhibitor-resistant non-small-cell lung cancer cells to erlotinib in vitro and in vivo.Br J Pharmacol. 2017 Oct;174(20):3608-3622. doi: 10.1111/bph.13961. Epub 2017 Aug 24. Br J Pharmacol. 2017. PMID: 28749535 Free PMC article.
-
Inhibition of JAK1/2 can overcome EGFR-TKI resistance in human NSCLC.Biochem Biophys Res Commun. 2020 Jun 18;527(1):305-310. doi: 10.1016/j.bbrc.2020.04.095. Epub 2020 May 11. Biochem Biophys Res Commun. 2020. PMID: 32446385
-
Design, synthesis and biological evaluation of novel heptamethine cyanine dye-erlotinib conjugates as antitumor agents.Bioorg Med Chem Lett. 2020 Dec 1;30(23):127557. doi: 10.1016/j.bmcl.2020.127557. Epub 2020 Sep 16. Bioorg Med Chem Lett. 2020. PMID: 32949719
-
Clinical evaluation of dacomitinib for the treatment of metastatic non-small cell lung cancer (NSCLC): current perspectives.Drug Des Devel Ther. 2019 Sep 6;13:3187-3198. doi: 10.2147/DDDT.S194231. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31564835 Free PMC article. Review.
-
First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.Cochrane Database Syst Rev. 2016 May 25;(5):CD010383. doi: 10.1002/14651858.CD010383.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD010383. doi: 10.1002/14651858.CD010383.pub3 PMID: 27223332 Updated. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

