High-Efficiency Expression and Purification of DNAJB6b Based on the pH-Modulation of Solubility and Denaturant-Modulation of Size
- PMID: 35056736
- PMCID: PMC8781954
- DOI: 10.3390/molecules27020418
High-Efficiency Expression and Purification of DNAJB6b Based on the pH-Modulation of Solubility and Denaturant-Modulation of Size
Abstract
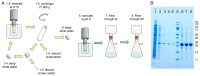
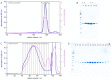
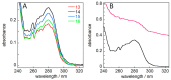
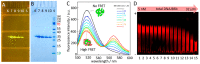
The chaperone DNAJB6b delays amyloid formation by suppressing the nucleation of amyloid fibrils and increases the solubility of amyloid-prone proteins. These dual effects on kinetics and equilibrium are related to the unusually high chemical potential of DNAJB6b in solution. As a consequence, the chaperone alone forms highly polydisperse oligomers, whereas in a mixture with an amyloid-forming protein or peptide it may form co-aggregates to gain a reduced chemical potential, thus enabling the amyloid peptide to increase its chemical potential leading to enhanced solubility of the peptide. Understanding such action at the level of molecular driving forces and detailed structures requires access to highly pure and sequence homogeneous DNAJB6b with no sequence extension. We therefore outline here an expression and purification protocol of the protein "as is" with no tags leading to very high levels of pure protein based on its physicochemical properties, including size and charge. The versatility of the protocol is demonstrated through the expression of an isotope labelled protein and seven variants, and the purification of three of these. The activity of the protein is bench-marked using aggregation assays. Two of the variants are used to produce a palette of fluorescent DNAJB6b labelled at an engineered N- or C-terminal cysteine.
Keywords: extraction; self-assembly; solubilization.
Conflict of interest statement
The author declares no conflict of interest.
Figures


Similar articles
-
The Ability of DNAJB6b to Suppress Amyloid Formation Depends on the Chaperone Aggregation State.ACS Chem Neurosci. 2024 May 1;15(9):1732-1737. doi: 10.1021/acschemneuro.4c00120. Epub 2024 Apr 19. ACS Chem Neurosci. 2024. PMID: 38640082 Free PMC article.
-
Unraveling the structure and dynamics of the human DNAJB6b chaperone by NMR reveals insights into Hsp40-mediated proteostasis.Proc Natl Acad Sci U S A. 2019 Oct 22;116(43):21529-21538. doi: 10.1073/pnas.1914999116. Epub 2019 Oct 7. Proc Natl Acad Sci U S A. 2019. PMID: 31591220 Free PMC article.
-
The HSP40 family chaperone isoform DNAJB6b prevents neuronal cells from tau aggregation.BMC Biol. 2023 Dec 18;21(1):293. doi: 10.1186/s12915-023-01798-6. BMC Biol. 2023. PMID: 38110916 Free PMC article.
-
Neuromuscular Diseases Due to Chaperone Mutations: A Review and Some New Results.Int J Mol Sci. 2020 Feb 19;21(4):1409. doi: 10.3390/ijms21041409. Int J Mol Sci. 2020. PMID: 32093037 Free PMC article. Review.
-
Impact of Amyloid Polymorphism on Prion-Chaperone Interactions in Yeast.Viruses. 2019 Apr 16;11(4):349. doi: 10.3390/v11040349. Viruses. 2019. PMID: 30995727 Free PMC article. Review.
Cited by
-
On the micelle formation of DNAJB6b.QRB Discov. 2023 Aug 15;4:e6. doi: 10.1017/qrd.2023.4. eCollection 2023. QRB Discov. 2023. PMID: 37593255 Free PMC article.
-
The Ability of DNAJB6b to Suppress Amyloid Formation Depends on the Chaperone Aggregation State.ACS Chem Neurosci. 2024 May 1;15(9):1732-1737. doi: 10.1021/acschemneuro.4c00120. Epub 2024 Apr 19. ACS Chem Neurosci. 2024. PMID: 38640082 Free PMC article.
-
Tertiary structure and conformational dynamics of the anti-amyloidogenic chaperone DNAJB6b at atomistic resolution.Nat Commun. 2024 Apr 16;15(1):3285. doi: 10.1038/s41467-024-46587-z. Nat Commun. 2024. PMID: 38627370 Free PMC article.
References
-
- Hageman J., Rujano M.A., van Waarde M.A., Kakkar V., Dirks R.P., Govorukhina N., Oosterveld-Hut H.M., Lubsen N.H., Kampinga H.H. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol. Cell. 2010;37:355–369. doi: 10.1016/j.molcel.2010.01.001. - DOI - PubMed
-
- Chien V., Aitken J.F., Zhang S., Buchanan C.M., Hickey A., Brittain T., Cooper G.J., Loomes K.M. The chaperone proteins HSP70, HSP40/DnaJ and GRP78/BiP suppress misfolding and formation of β-sheet-containing aggregates by human amylin: A potential role for defective chaperone biology in Type 2 diabetes. Biochem. J. 2010;432:113–121. doi: 10.1042/BJ20100434. - DOI - PubMed
-
- Gillis J., Schipper-Krom S., Juenemann K., Gruber A., Coolen S., van den Nieuwendijk R., van Veen H., Overkleeft H., Goedhart J., Kampinga H.H., et al. The DNAJB6 and DNAJB8 protein chaperones prevent intracellular aggregation of polyglutamine repeats. J. Biol. Chem. 2013;288:17225–17237. doi: 10.1074/jbc.M112.421685. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

