Catabolism of Hydroxyproline in Vertebrates: Physiology, Evolution, Genetic Diseases and New siRNA Approach for Treatment
- PMID: 35055190
- PMCID: PMC8779045
- DOI: 10.3390/ijms23021005
Catabolism of Hydroxyproline in Vertebrates: Physiology, Evolution, Genetic Diseases and New siRNA Approach for Treatment
Abstract
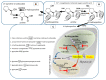
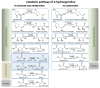
Hydroxyproline is one of the most prevalent amino acids in animal proteins. It is not a genetically encoded amino acid, but, rather, it is produced by the post-translational modification of proline in collagen, and a few other proteins, by prolyl hydroxylase enzymes. Although this post-translational modification occurs in a limited number of proteins, its biological significance cannot be overestimated. Considering that hydroxyproline cannot be re-incorporated into pro-collagen during translation, it should be catabolized following protein degradation. A cascade of reactions leads to production of two deleterious intermediates: glyoxylate and hydrogen peroxide, which need to be immediately converted. As a result, the enzymes involved in hydroxyproline catabolism are located in specific compartments: mitochondria and peroxisomes. The particular distribution of catabolic enzymes in these compartments, in different species, depends on their dietary habits. Disturbances in hydroxyproline catabolism, due to genetic aberrations, may lead to a severe disease (primary hyperoxaluria), which often impairs kidney function. The basis of this condition is accumulation of glyoxylate and its conversion to oxalate. Since calcium oxalate is insoluble, children with this rare inherited disorder suffer from progressive kidney damage. This condition has been nearly incurable until recently, as significant advances in substrate reduction therapy using small interference RNA led to a breakthrough in primary hyperoxaluria type 1 treatment.
Keywords: HIF-1α; collagen post-translational modification; glyoxylate; hydroxyproline; oxalate; primary hyperoxaluria; prolyl hydroxylase; protein compartmentalization; small interference RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Primary hyperoxalurias: disorders of glyoxylate detoxification.Biochim Biophys Acta. 2012 Sep;1822(9):1453-64. doi: 10.1016/j.bbadis.2012.03.004. Epub 2012 Mar 14. Biochim Biophys Acta. 2012. PMID: 22446032 Review.
-
Mitochondrial hydroxyproline metabolism: implications for primary hyperoxaluria.Am J Nephrol. 2005 Mar-Apr;25(2):171-5. doi: 10.1159/000085409. Epub 2005 Apr 21. Am J Nephrol. 2005. PMID: 15849464 Free PMC article.
-
Metabolism of (13)C5-hydroxyproline in mouse models of Primary Hyperoxaluria and its inhibition by RNAi therapeutics targeting liver glycolate oxidase and hydroxyproline dehydrogenase.Biochim Biophys Acta. 2016 Feb;1862(2):233-9. doi: 10.1016/j.bbadis.2015.12.001. Epub 2015 Dec 2. Biochim Biophys Acta. 2016. PMID: 26655602 Free PMC article.
-
4-Hydroxyproline metabolism and glyoxylate production: A target for substrate depletion in primary hyperoxaluria?Kidney Int. 2006 Dec;70(11):1891-3. doi: 10.1038/sj.ki.5001987. Kidney Int. 2006. PMID: 17130820
-
Molecular basis of primary hyperoxaluria: clues to innovative treatments.Urolithiasis. 2019 Feb;47(1):67-78. doi: 10.1007/s00240-018-1089-z. Epub 2018 Nov 14. Urolithiasis. 2019. PMID: 30430197 Review.
Cited by
-
Amino Acids in Health and Disease: The Good, the Bad, and the Ugly.Int J Mol Sci. 2023 Mar 3;24(5):4931. doi: 10.3390/ijms24054931. Int J Mol Sci. 2023. PMID: 36902358 Free PMC article.
-
Protein catabolites as blood-based biomarkers of aging physiology: Findings from the Dog Aging Project.bioRxiv [Preprint]. 2024 Oct 21:2024.10.17.618956. doi: 10.1101/2024.10.17.618956. bioRxiv. 2024. PMID: 39484426 Free PMC article. Preprint.
-
Collagen constitutes about 12% in females and 17% in males of the total protein in mice.Sci Rep. 2023 Mar 18;13(1):4490. doi: 10.1038/s41598-023-31566-z. Sci Rep. 2023. PMID: 36934197 Free PMC article.
-
Transcriptome Analysis Reveals the Potential Key Genes in Nutritional Deposition in the Common Carp (Cyprinus carpio).Animals (Basel). 2024 Jun 30;14(13):1939. doi: 10.3390/ani14131939. Animals (Basel). 2024. PMID: 38998051 Free PMC article.
References
-
- Etherington D.J., Pugh D., Silver I.A. Collagen degradation in an experimental inflammatory lesion: Studies on the role of the macrophage. Acta. Biol. Med. Ger. 1981;40:1625–1636. - PubMed
-
- Buchalski B., Wood K.D., Challa A., Fargue S., Holmes R.P., Lowther W.T., Knight J. The effects of the inactivation of Hydroxyproline dehydrogenase on urinary oxalate and glycolate excretion in mouse models of primary hyperoxaluria. Biochim. Biophys. Acta Mol. Basis. Dis. 2020;1866:165633. doi: 10.1016/j.bbadis.2019.165633. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

