Development of Cobalt-Binding Peptide Chelate from Human Serum Albumin: Cobalt-Binding Properties and Stability
- PMID: 35054904
- PMCID: PMC8775498
- DOI: 10.3390/ijms23020719
Development of Cobalt-Binding Peptide Chelate from Human Serum Albumin: Cobalt-Binding Properties and Stability
Abstract
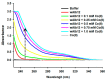
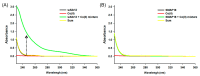
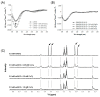
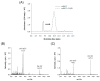
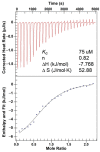
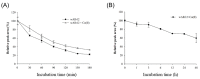
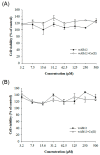
Radioactive isotopes are used as drugs or contrast agents in the medical field after being conjugated with chelates such as DOTA, NOTA, DTPA, TETA, CyDTA, TRITA, and DPDP. The N-terminal sequence of human serum albumin (HSA) is known as a metal binding site, such as for Co2+, Cu2+, and Ni2+. For this study, we designed and synthesized wAlb12 peptide from the N-terminal region of HSA, which can bind to cobalt, to develop a peptide-based chelate. The wAlb12 with a random coil structure tightly binds to the Co(II) ion. Moreover, the binding property of wAlb12 toward Co(II) was confirmed using various spectroscopic experiments. To identify the binding site of wAlb12, the analogs were synthesized by alanine scanning mutagenesis. Among them, H3A and Ac-wAlb12 did not bind to Co(II). The analysis of the binding regions confirmed that the His3 and α-amino group of the N-terminal region are important for Co(II) binding. The wAlb12 bound to Co(II) with Kd of 75 μM determined by isothermal titration calorimetry when analyzed by a single-site binding model. For the use of wAlb12 as a chelate in humans, its cytotoxicity and stability were investigated. Trypsin stability showed that the wAlb12 - Co(II) complex was more stable than wAlb12 alone. Furthermore, the cell viability analysis showed wAlb12 and wAlb12 + Co(II) to be non-toxic to the Raw 264.7 and HEK 293T cell lines. Therefore, a hot radioactive isotope such as cobalt-57 will have the same effect as a stable isotope cobalt. Accordingly, we expect wAlb12 to be used as a peptide chelate that binds with radioactive isotopes.
Keywords: cobalt binding; peptides; stability; structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Characterization of the Co(2+) and Ni(2+) binding amino-acid residues of the N-terminus of human albumin. An insight into the mechanism of a new assay for myocardial ischemia.Eur J Biochem. 2001 Jan;268(1):42-7. doi: 10.1046/j.1432-1327.2001.01846.x. Eur J Biochem. 2001. PMID: 11121100
-
Spectroscopic and thermodynamic determination of three distinct binding sites for Co(II) ions in human serum albumin.J Inorg Biochem. 2009 Jul;103(7):1005-13. doi: 10.1016/j.jinorgbio.2009.04.011. Epub 2009 May 3. J Inorg Biochem. 2009. PMID: 19487034
-
Ischemia-modified albumin: Crosstalk between fatty acid and cobalt binding.Prostaglandins Leukot Essent Fatty Acids. 2018 Aug;135:147-157. doi: 10.1016/j.plefa.2018.07.014. Epub 2018 Jul 20. Prostaglandins Leukot Essent Fatty Acids. 2018. PMID: 30103926 Free PMC article. Review.
-
Synergistic effects of metal-induced aggregation of human serum albumin.Colloids Surf B Biointerfaces. 2019 Jan 1;173:751-758. doi: 10.1016/j.colsurfb.2018.10.061. Epub 2018 Oct 23. Colloids Surf B Biointerfaces. 2019. PMID: 30384272
-
Evidence that the principal CoII-binding site in human serum albumin is not at the N-terminus: implication on the albumin cobalt binding test for detecting myocardial ischemia.Biochemistry. 2007 Feb 27;46(8):2267-74. doi: 10.1021/bi061783p. Epub 2007 Feb 3. Biochemistry. 2007. PMID: 17274600
Cited by
-
Human Serum Albumin: From Molecular Aspects to Biotechnological Applications.Int J Mol Sci. 2023 Feb 17;24(4):4081. doi: 10.3390/ijms24044081. Int J Mol Sci. 2023. PMID: 36835490 Free PMC article.
-
Cobalt Oxide Nanoparticle Synthesis by Cell-Surface-Engineered Recombinant Escherichia coli and Potential Application for Anticancer Treatment.ACS Omega. 2024 Jul 9;9(29):31373-31383. doi: 10.1021/acsomega.3c10246. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072137 Free PMC article.
References
-
- Wojcieszynski A.P., Hill P.M., Rosenberg S.A., Hullett C.R., Labby Z.E., Paliwal B., Geurts M.W., Bayliss R.A., Bayouth J.E., Harari P.M. Dosimetric comparison of real-time MRI-guided tri-cobalt-60 versus linear accelerator-based stereotactic body radiation therapy lung cancer plans. Technol. Cancer Res. Treat. 2017;16:366–372. doi: 10.1177/1533034617691407. - DOI - PMC - PubMed
-
- Gourni E., Del Pozzo L., Kheirallah E., Smerling C., Waser B., Reubi J.-C., Paterson B.M., Donnelly P.S., Meyer P.T., Maecke H.R. Copper-64 labeled macrobicyclic sarcophagine coupled to a GRP receptor antagonist shows great promise for PET imaging of prostate cancer. Mol. Pharm. 2015;12:2781–2790. doi: 10.1021/mp500671j. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

