Pathophysiology of Lipid Droplets in Neuroglia
- PMID: 35052526
- PMCID: PMC8773017
- DOI: 10.3390/antiox11010022
Pathophysiology of Lipid Droplets in Neuroglia
Abstract
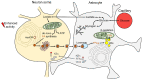
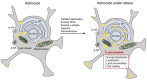
In recent years, increasing evidence regarding the functional importance of lipid droplets (LDs), cytoplasmic storage organelles in the central nervous system (CNS), has emerged. Although not abundantly present in the CNS under normal conditions in adulthood, LDs accumulate in the CNS during development and aging, as well as in some neurologic disorders. LDs are actively involved in cellular lipid turnover and stress response. By regulating the storage of excess fatty acids, cholesterol, and ceramides in addition to their subsequent release in response to cell needs and/or environmental stressors, LDs are involved in energy production, in the synthesis of membranes and signaling molecules, and in the protection of cells against lipotoxicity and free radicals. Accumulation of LDs in the CNS appears predominantly in neuroglia (astrocytes, microglia, oligodendrocytes, ependymal cells), which provide trophic, metabolic, and immune support to neuronal networks. Here we review the most recent findings on the characteristics and functions of LDs in neuroglia, focusing on astrocytes, the key homeostasis-providing cells in the CNS. We discuss the molecular mechanisms affecting LD turnover in neuroglia under stress and how this may protect neural cell function. We also highlight the role (and potential contribution) of neuroglial LDs in aging and in neurologic disorders.
Keywords: astrocytes; lipid droplets; metabolic and oxidative stress; neuroglia; neurologic disorders; pathophysiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Astrocytes in stress accumulate lipid droplets.Glia. 2021 Jun;69(6):1540-1562. doi: 10.1002/glia.23978. Epub 2021 Feb 20. Glia. 2021. PMID: 33609060 Free PMC article.
-
Lipid droplets and fatty acid-induced lipotoxicity: in a nutshell.FEBS Lett. 2024 May;598(10):1207-1214. doi: 10.1002/1873-3468.14808. Epub 2024 Jan 28. FEBS Lett. 2024. PMID: 38281809
-
Friend or Foe: Lipid Droplets as Organelles for Protein and Lipid Storage in Cellular Stress Response, Aging and Disease.Molecules. 2020 Oct 30;25(21):5053. doi: 10.3390/molecules25215053. Molecules. 2020. PMID: 33143278 Free PMC article. Review.
-
Hepatic lipid droplets: A balancing act between energy storage and metabolic dysfunction in NAFLD.Mol Metab. 2021 Aug;50:101115. doi: 10.1016/j.molmet.2020.101115. Epub 2020 Nov 10. Mol Metab. 2021. PMID: 33186758 Free PMC article. Review.
-
Lipid droplets and lipid mediators in viral infection and immunity.FEMS Microbiol Rev. 2021 Aug 17;45(4):fuaa066. doi: 10.1093/femsre/fuaa066. FEMS Microbiol Rev. 2021. PMID: 33512504 Free PMC article. Review.
Cited by
-
FABP7: a glial integrator of sleep, circadian rhythms, plasticity, and metabolic function.Front Syst Neurosci. 2023 Jun 19;17:1212213. doi: 10.3389/fnsys.2023.1212213. eCollection 2023. Front Syst Neurosci. 2023. PMID: 37404868 Free PMC article.
-
Transcriptional programs mediating neuronal toxicity and altered glial-neuronal signaling in a Drosophila knock-in tauopathy model.Genome Res. 2024 May 15;34(4):590-605. doi: 10.1101/gr.278576.123. Genome Res. 2024. PMID: 38599684 Free PMC article.
-
Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles.Nat Rev Neurosci. 2023 Aug;24(8):474-486. doi: 10.1038/s41583-023-00711-y. Epub 2023 May 31. Nat Rev Neurosci. 2023. PMID: 37258632 Review.
-
A Perspective on the Link between Mitochondria-Associated Membranes (MAMs) and Lipid Droplets Metabolism in Neurodegenerative Diseases.Biology (Basel). 2023 Mar 8;12(3):414. doi: 10.3390/biology12030414. Biology (Basel). 2023. PMID: 36979106 Free PMC article. Review.
-
Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress.Nat Neurosci. 2024 Oct;27(10):1918-1933. doi: 10.1038/s41593-024-01740-1. Epub 2024 Aug 26. Nat Neurosci. 2024. PMID: 39187706
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

