Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients?
- PMID: 35047684
- PMCID: PMC8749262
- DOI: 10.1136/bmjos-2019-100013
Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients?
Abstract
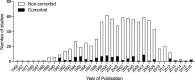
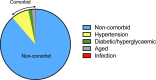
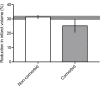
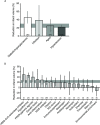
Stroke is a significant cause of mortality and morbidity for which there are limited treatment options. Virtually all drug interventions that have been successful preclinically in experimental stroke have failed to translate to an effective treatment in the clinical setting. In this review, we examine one of the factors likely contributing to this lack of translation, the failure of preclinical studies to consider fully the advanced age and comorbidities (eg, hypertension or diabetes) present in most patients with stroke. Age and comorbidities affect the likelihood of suffering a stroke, disease progression and the response to treatment. Analysing data from preclinical systematic reviews of interventions for ischaemic stroke we show that only 11.4% of studies included an aged or comorbid model, with hypertension being the most frequent. The degree of protection (% reduction in infarct volume) varied depending on the comorbidity and the type of intervention. We consider reasons for the lack of attention to comorbid and aged animals in stroke research and discuss the value of testing a potential therapy in models representing a range of comorbidities that affect patients with stroke. These models can help establish any limits to a treatment's efficacy and inform the design of clinical trials in appropriate patient populations.
Keywords: aged; comorbidity; ischaemia; preclinical; stroke.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Impact of aging and comorbidities on ischemic stroke outcomes in preclinical animal models: A translational perspective.Exp Neurol. 2021 Jan;335:113494. doi: 10.1016/j.expneurol.2020.113494. Epub 2020 Oct 7. Exp Neurol. 2021. PMID: 33035516 Free PMC article. Review.
-
How has the impact of 'care pathway technologies' on service integration in stroke care been measured and what is the strength of the evidence to support their effectiveness in this respect?Int J Evid Based Healthc. 2008 Mar;6(1):78-110. doi: 10.1111/j.1744-1609.2007.00098.x. Int J Evid Based Healthc. 2008. PMID: 21631815
-
Ablation: Atrial fibrillation: diagnosis and management: Network meta-analysis J2.London: National Institute for Health and Care Excellence (NICE); 2021 Apr. London: National Institute for Health and Care Excellence (NICE); 2021 Apr. PMID: 34165931 Free Books & Documents. Review.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.Health Technol Assess. 2009 Sep;13(41):1-190, 215-357, iii-iv. doi: 10.3310/hta13410. Health Technol Assess. 2009. PMID: 19726018 Review.
Cited by
-
Phagocytic microglia and macrophages in brain injury and repair.CNS Neurosci Ther. 2022 Sep;28(9):1279-1293. doi: 10.1111/cns.13899. Epub 2022 Jun 25. CNS Neurosci Ther. 2022. PMID: 35751629 Free PMC article. Review.
-
A practical guide to preclinical systematic review and meta-analysis.Pain. 2020 Sep 1;161(9):1949-1954. doi: 10.1097/j.pain.0000000000001974. Pain. 2020. PMID: 33449500 Free PMC article. No abstract available.
-
Spontaneously Hypertensive Rats Present Exacerbated Focal Stroke Behavioral Outcomes.Brain Sci. 2024 Aug 21;14(8):838. doi: 10.3390/brainsci14080838. Brain Sci. 2024. PMID: 39199529 Free PMC article.
-
Akkermansia muciniphila for the Prevention of Type 2 Diabetes and Obesity: A Meta-Analysis of Animal Studies.Nutrients. 2024 Oct 11;16(20):3440. doi: 10.3390/nu16203440. Nutrients. 2024. PMID: 39458436 Free PMC article.
-
A multi-disciplinary commentary on preclinical research to investigate vascular contributions to dementia.Cereb Circ Cogn Behav. 2023 Oct 11;5:100189. doi: 10.1016/j.cccb.2023.100189. eCollection 2023. Cereb Circ Cogn Behav. 2023. PMID: 37941765 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
