A Clinical Tool to Guide Selection and Utilization of Marginal Donor Livers With Graft Steatosis in Liver Transplantation
- PMID: 35047662
- PMCID: PMC8759620
- DOI: 10.1097/TXD.0000000000001280
A Clinical Tool to Guide Selection and Utilization of Marginal Donor Livers With Graft Steatosis in Liver Transplantation
Abstract
Background: Donor liver biopsy (DLBx) in liver transplantation provides information on allograft quality; however, predicting outcomes from these allografts remains difficult.
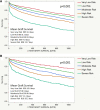
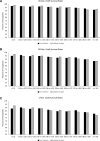
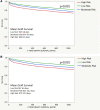
Methods: Between 2006 and 2015, 16 691 transplants with DLBx were identified from the Standard Transplant Analysis and Research database. Cox proportional hazard regression analyses identified donor and recipient characteristics associated with 30-d, 90-d, 1-y, and 3-y graft survival. A composite model, the Liver Transplant After Biopsy (LTAB) score, was created. The Mini-LTAB was then derived consisting of only donor age, macrosteatosis on DLBx, recipient model for end-stage liver disease score, and cold ischemic time. Risk groups were identified for each score and graft survival was evaluated. P values <0.05 were considered significant.
Results: The LTAB model used 14 variables and 5 risk groups and identified low-, mild-, moderate-, high-, and severe-risk groups. Compared with moderate-risk recipients, severe-risk recipients had increased risk of graft loss at 30 d (hazard ratio, 3.270; 95% confidence interval, 2.568-4.120) and at 1 y (2.258; 1.928-2.544). The Mini-LTAB model identified low-, moderate-, and high-risk groups. Graft survival in Mini-LTAB high-risk transplants was significantly lower than moderate- or low-risk transplants at all time points.
Conclusions: The LTAB and Mini-LTAB scores represent guiding principles and provide clinically useful tools for the successful selection and utilization of marginal allografts in liver transplantation.
Copyright © 2022 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Figures


Similar articles
-
Liver Transplant Survival Index for Patients with Model for End-Stage Liver Disease Score ≥ 35: Modeling Risk and Adjusting Expectations in the Share 35 Era.J Am Coll Surg. 2019 Apr;228(4):437-450.e8. doi: 10.1016/j.jamcollsurg.2018.12.009. Epub 2018 Dec 27. J Am Coll Surg. 2019. PMID: 30594593
-
Higher thresholds for the utilization of steatotic allografts in liver transplantation: Analysis from a U.S. national database.PLoS One. 2020 Apr 2;15(4):e0230995. doi: 10.1371/journal.pone.0230995. eCollection 2020. PLoS One. 2020. PMID: 32240235 Free PMC article.
-
The UK DCD Risk Score: A new proposal to define futility in donation-after-circulatory-death liver transplantation.J Hepatol. 2018 Mar;68(3):456-464. doi: 10.1016/j.jhep.2017.10.034. Epub 2017 Nov 15. J Hepatol. 2018. PMID: 29155020
-
Changing landscape of liver transplant in the United States-time for a new innovative way to define and utilize the "non-standard liver allograft"-a proposal.Front Transplant. 2024 Aug 8;3:1449407. doi: 10.3389/frtra.2024.1449407. eCollection 2024. Front Transplant. 2024. PMID: 39176402 Free PMC article. Review.
-
Donor liver steatosis and graft selection for liver transplantation: a short review.Eur Rev Med Pharmacol Sci. 2005 Sep-Oct;9(5):295-7. Eur Rev Med Pharmacol Sci. 2005. PMID: 16231593 Review.
Cited by
-
How to Preserve Steatotic Liver Grafts for Transplantation.J Clin Med. 2023 Jun 12;12(12):3982. doi: 10.3390/jcm12123982. J Clin Med. 2023. PMID: 37373676 Free PMC article. Review.
-
Steatotic Donor Transplant Livers: Preservation Strategies to Mitigate against Ischaemia-Reperfusion Injury.Int J Mol Sci. 2024 Apr 24;25(9):4648. doi: 10.3390/ijms25094648. Int J Mol Sci. 2024. PMID: 38731866 Free PMC article. Review.
References
-
- Baccarani U, Adani GL, Isola M, et al. . Steatosis of the graft is a risk factor for posttransplantation biliary complications. Transplant Proc. 2009;41:1313–1315. - PubMed
-
- Spitzer AL, Lao OB, Dick AA, et al. . The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16:874–884. - PubMed
-
- McCormack L, Petrowsky H, Jochum W, et al. . Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246:940–946. - PubMed
LinkOut - more resources
Full Text Sources

