Effects of tRNA-derived fragments and microRNAs regulatory network on pancreatic acinar intracellular trypsinogen activation
- PMID: 35045793
- PMCID: PMC8973995
- DOI: 10.1080/21655979.2021.2018880
Effects of tRNA-derived fragments and microRNAs regulatory network on pancreatic acinar intracellular trypsinogen activation
Abstract
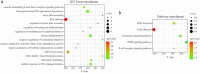
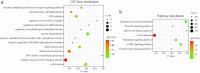
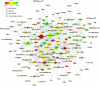
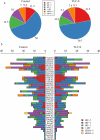
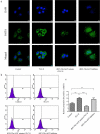
Acute pancreatitis (AP) is a common gastrointestinal disease with substantial morbidity and mortality. Pancreatic acinar intracellular trypsinogen activation (PAITA) is an important event in the early stage of AP. The present study aimed to investigate the effects of tRNA-derived fragments (tRFs) and the microRNA regulatory network on pancreatic acinar intracellular trypsinogen activation (PAITA) and identify novel key targets in AP. Taurolithocholic acid 3-sulfate (TLC-S)-treated AR42J cells were used to establish a PAITA model. Twenty differentially expressed tRFs and 35 DE microRNAs were identified in PAITA through gene sequencing. Based on these genes, we established the tRF-mRNA and microRNA-mRNA regulatory networks by using bioinformatics methods. The networks revealed 29 hub microRNAs (e.g., Let-7 family, miR-21-3p.) and 19 hub tRFs (e.g., tRF3-Thr-AGT, i-tRF-Met-CAT) in PAITA. GO analysis showed that the functions of the two networks were similar and mainly enriched in RNA splicing, mRNA processing, and so on. tRF3-Thr-AGT, targeting Btg2, Cd44, Zbp1, etc., was significantly decreased in PAITA. Moreover, the trypsinogen activation level was increased significantly in the tRF3-Thr-AGT deficiency groups, but rescued by tRF3-Thr-AGT mimics. The results revealed that downregulated tRF3-Thr-AGT was involved in PAITA. This study provides potential novel targets for researching the underlying mechanisms of AP.
Keywords: Acute pancreatitis; small non-coding RNA; tRF3-THr-AGT; tRNA-derived fragments; trypsinogen activation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Effects of Egr1 on pancreatic acinar intracellular trypsinogen activation and the associated ceRNA network.Mol Med Rep. 2020 Sep;22(3):2496-2506. doi: 10.3892/mmr.2020.11316. Epub 2020 Jul 9. Mol Med Rep. 2020. PMID: 32705196 Free PMC article.
-
Endogenous tRNA-derived small RNA (tRF3-Thr-AGT) inhibits ZBP1/NLRP3 pathway-mediated cell pyroptosis to attenuate acute pancreatitis (AP).J Cell Mol Med. 2021 Nov;25(22):10441-10453. doi: 10.1111/jcmm.16972. Epub 2021 Oct 13. J Cell Mol Med. 2021. PMID: 34643045 Free PMC article.
-
Role of the c-Jun N-terminal kinase signaling pathway in the activation of trypsinogen in rat pancreatic acinar cells.Int J Mol Med. 2018 Feb;41(2):1119-1126. doi: 10.3892/ijmm.2017.3266. Epub 2017 Nov 17. Int J Mol Med. 2018. PMID: 29207022
-
Pathogenic mechanisms of acute pancreatitis.Curr Opin Gastroenterol. 2012 Sep;28(5):507-15. doi: 10.1097/MOG.0b013e3283567f52. Curr Opin Gastroenterol. 2012. PMID: 22885948 Free PMC article. Review.
-
Roles of Autophagy and Pancreatic Secretory Trypsin Inhibitor in Trypsinogen Activation in Acute Pancreatitis.Pancreas. 2020 Apr;49(4):493-497. doi: 10.1097/MPA.0000000000001519. Pancreas. 2020. PMID: 32282761 Review.
Cited by
-
ZBP1: A Powerful Innate Immune Sensor and Double-Edged Sword in Host Immunity.Int J Mol Sci. 2022 Sep 6;23(18):10224. doi: 10.3390/ijms231810224. Int J Mol Sci. 2022. PMID: 36142136 Free PMC article. Review.
-
Biological function and clinical application prospect of tsRNAs in digestive system biology and pathology.Cell Commun Signal. 2023 Oct 30;21(1):302. doi: 10.1186/s12964-023-01341-8. Cell Commun Signal. 2023. PMID: 37904174 Free PMC article. Review.
-
tRNA-derived fragments: mechanism of gene regulation and clinical application in lung cancer.Cell Oncol (Dordr). 2024 Feb;47(1):37-54. doi: 10.1007/s13402-023-00864-z. Epub 2023 Aug 29. Cell Oncol (Dordr). 2024. PMID: 37642916 Review.
-
Differential expression profiling of tRNA-Derived small RNAs and their potential roles in methamphetamine self-administered rats.Front Genet. 2023 Feb 2;14:1088498. doi: 10.3389/fgene.2023.1088498. eCollection 2023. Front Genet. 2023. PMID: 36845381 Free PMC article.
-
Transfer RNA-derived fragments as novel biomarkers of the onset and progression of gastric cancer.Exp Biol Med (Maywood). 2023 Jul;248(13):1095-1102. doi: 10.1177/15353702231179415. Epub 2023 Jun 30. Exp Biol Med (Maywood). 2023. PMID: 37387464 Free PMC article.
References
-
- Koutroumpakis E, Slivka A, Furlan A, et al. Management and outcomes of acute pancreatitis patients over the last decade: a US tertiary-center experience. Pancreatology. 2017;17(1):32–40. - PubMed
-
- Qin T, Fu Q, Pan YF, et al. Expressions of miR-22 and miR-135a in acute pancreatitis. J Huazhong Univ Sci Technolog Med Sci. 2014;34(2):225–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
