Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England
- PMID: 35045566
- PMCID: PMC9018410
- DOI: 10.1038/s41591-022-01699-1
Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England
Abstract
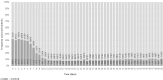
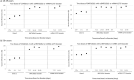
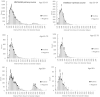
Booster vaccination with messenger RNA (mRNA) vaccines has been offered to adults in England starting on 14 September 2021. We used a test-negative case-control design to estimate the relative effectiveness of a booster dose of BNT162b2 (Pfizer-BioNTech) compared to only a two-dose primary course (at least 175 days after the second dose) or unvaccinated individuals from 13 September 2021 to 5 December 2021, when Delta variant was dominant in circulation. Outcomes were symptomatic coronavirus disease 2019 (COVID-19) and hospitalization. The relative effectiveness against symptomatic disease 14-34 days after a BNT162b2 or mRNA-1273 (Moderna) booster after a ChAdOx1-S (AstraZeneca) and BNT162b2 as a primary course ranged from around 85% to 95%. Absolute vaccine effectiveness ranged from 94% to 97% and was similar in all age groups. Limited waning was seen 10 or more weeks after the booster. Against hospitalization or death, absolute effectiveness of a BNT162b2 booster ranged from around 97% to 99% in all age groups irrespective of the primary course, with no evidence of waning up to 10 weeks. This study provides real-world evidence of substantially increased protection from the booster vaccine dose against mild and severe disease irrespective of the primary course.
© 2022. Crown.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study.BMJ. 2021 May 13;373:n1088. doi: 10.1136/bmj.n1088. BMJ. 2021. PMID: 33985964 Free PMC article.
-
Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant.N Engl J Med. 2022 Apr 21;386(16):1532-1546. doi: 10.1056/NEJMoa2119451. Epub 2022 Mar 2. N Engl J Med. 2022. PMID: 35249272 Free PMC article.
-
Comparative effectiveness of different primary vaccination courses on mRNA-based booster vaccines against SARs-COV-2 infections: a time-varying cohort analysis using trial emulation in the Virus Watch community cohort.Int J Epidemiol. 2023 Apr 19;52(2):342-354. doi: 10.1093/ije/dyad002. Int J Epidemiol. 2023. PMID: 36655537 Free PMC article.
-
Vaccinating against a Novel Pathogen: A Critical Review of COVID-19 Vaccine Effectiveness Evidence.Microorganisms. 2023 Dec 31;12(1):89. doi: 10.3390/microorganisms12010089. Microorganisms. 2023. PMID: 38257917 Free PMC article. Review.
-
The efficacy and effectiveness of COVID-19 vaccines around the world: a mini-review and meta-analysis.Ann Clin Microbiol Antimicrob. 2023 May 19;22(1):42. doi: 10.1186/s12941-023-00594-y. Ann Clin Microbiol Antimicrob. 2023. PMID: 37208749 Free PMC article. Review.
Cited by
-
Protective efficacy of COVAXIN® against Delta and Omicron variants in hamster model.iScience. 2022 Oct 21;25(10):105178. doi: 10.1016/j.isci.2022.105178. Epub 2022 Sep 22. iScience. 2022. PMID: 36164480 Free PMC article.
-
Possible opportunities and challenges for traditional Chinese medicine research in 2035.Front Pharmacol. 2024 Jun 21;15:1426300. doi: 10.3389/fphar.2024.1426300. eCollection 2024. Front Pharmacol. 2024. PMID: 38974044 Free PMC article.
-
A SARS-CoV-2 RBD vaccine fused to the chemokine MIP-3α elicits sustained murine antibody responses over 12 months and enhanced lung T-cell responses.Front Immunol. 2024 Feb 2;15:1292059. doi: 10.3389/fimmu.2024.1292059. eCollection 2024. Front Immunol. 2024. PMID: 38370404 Free PMC article.
-
Understanding COVID-19 Vaccine Effectiveness Against Death Using a Novel Measure: COVID Excess Mortality Percentage.Res Sq [Preprint]. 2022 Dec 16:rs.3.rs-2359020. doi: 10.21203/rs.3.rs-2359020/v1. Res Sq. 2022. Update in: Vaccines (Basel). 2023 Feb 07;11(2):379. doi: 10.3390/vaccines11020379. PMID: 36561183 Free PMC article. Updated. Preprint.
-
Comparative effectiveness of BNT162b2 versus mRNA-1273 covid-19 vaccine boosting in England: matched cohort study in OpenSAFELY-TPP.BMJ. 2023 Mar 15;380:e072808. doi: 10.1136/bmj-2022-072808. BMJ. 2023. PMID: 36921925 Free PMC article.
References
-
- Pritchard E, et al. Impact of vaccination on SARS-CoV-2 cases in the community: a population-based study using the UK’s COVID-19 infection survey. Nat. Med. 2021;27:370–1378. doi: 10.1038/s41591-021-01410-w. - DOI
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

