Bacterial peptidoglycan muropeptides benefit mitochondrial homeostasis and animal physiology by acting as ATP synthase agonists
- PMID: 35045336
- PMCID: PMC8825754
- DOI: 10.1016/j.devcel.2021.12.016
Bacterial peptidoglycan muropeptides benefit mitochondrial homeostasis and animal physiology by acting as ATP synthase agonists
Abstract
The symbiotic relationship between commensal microbes and host animals predicts unidentified beneficial impacts of individual bacterial metabolites on animal physiology. Peptidoglycan fragments (muropeptides) from the bacterial cell wall are known for their roles in pathogenicity and for inducing host immune responses. However, the potential beneficial usage of muropeptides from commensal bacteria by the host needs exploration. We identified a striking role for muropeptides in supporting mitochondrial homeostasis, development, and behaviors in Caenorhabditis elegans. We determined that the beneficial molecules are disaccharide muropeptides containing a short AA chain, and they enter intestinal-cell mitochondria to repress oxidative stress. Further analyses indicate that muropeptides execute this role by binding to and promoting the activity of ATP synthase. Therefore, given the exceptional structural conservation of ATP synthase, the role of muropeptides as a rare agonist of the ATP synthase presents a major conceptual modification regarding the impact of bacterial cell metabolites on animal physiology.
Keywords: ATP synthase; ATP synthase agonist; PG fragments; PGN; UPRmt; bacteria cell wall; food avoidance; mitochondrial stress; muropeptides; unfolded protein response.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The University of Colorado has filed a provisional patent application partly based on results from this study (63/231,350).
Figures
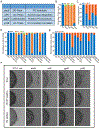
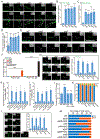

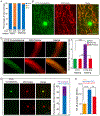

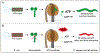
Similar articles
-
Escherichia coli CadB is capable of promiscuously transporting muropeptides and contributing to peptidoglycan recycling.J Bacteriol. 2024 Jan 25;206(1):e0036923. doi: 10.1128/jb.00369-23. Epub 2024 Jan 3. J Bacteriol. 2024. PMID: 38169298 Free PMC article.
-
Hijacking the Peptidoglycan Recycling Pathway of Escherichia coli to Produce Muropeptides.Chemistry. 2023 Jan 27;29(6):e202202991. doi: 10.1002/chem.202202991. Epub 2022 Dec 5. Chemistry. 2023. PMID: 36256497 Free PMC article.
-
Microbial Siderophore Enterobactin Promotes Mitochondrial Iron Uptake and Development of the Host via Interaction with ATP Synthase.Cell. 2018 Oct 4;175(2):571-582.e11. doi: 10.1016/j.cell.2018.07.032. Epub 2018 Aug 23. Cell. 2018. PMID: 30146159
-
Bacterial peptidoglycan degrading enzymes and their impact on host muropeptide detection.J Innate Immun. 2009;1(2):88-97. doi: 10.1159/000181181. J Innate Immun. 2009. PMID: 19319201 Free PMC article. Review.
-
Messenger functions of the bacterial cell wall-derived muropeptides.Biochemistry. 2012 Apr 10;51(14):2974-90. doi: 10.1021/bi300174x. Epub 2012 Mar 27. Biochemistry. 2012. PMID: 22409164 Free PMC article. Review.
Cited by
-
Microbiota-derived metabolites in regulating the development and physiology of Caenorhabditis elegans.Front Microbiol. 2023 Feb 28;14:1035582. doi: 10.3389/fmicb.2023.1035582. eCollection 2023. Front Microbiol. 2023. PMID: 36925470 Free PMC article. Review.
-
Bacterial peptidoglycan acts as a digestive signal mediating host adaptation to diverse food resources in C. elegans.Nat Commun. 2024 Apr 16;15(1):3286. doi: 10.1038/s41467-024-47530-y. Nat Commun. 2024. PMID: 38627398 Free PMC article.
-
Heat-inactivated Bifidobacterium adolescentis ameliorates colon senescence through Paneth-like-cell-mediated stem cell activation.Nat Commun. 2023 Sep 30;14(1):6121. doi: 10.1038/s41467-023-41827-0. Nat Commun. 2023. PMID: 37777508 Free PMC article.
-
Multi-Omics Integrative Analysis to Reveal the Impacts of Shewanella algae on the Development and Lifespan of Marine Nematode Litoditis marina.Int J Mol Sci. 2024 Aug 22;25(16):9111. doi: 10.3390/ijms25169111. Int J Mol Sci. 2024. PMID: 39201797 Free PMC article.
-
The hidden base of the iceberg: gut peptidoglycome dynamics is foundational to its influence on the host.Gut Microbes. 2024 Jan-Dec;16(1):2395099. doi: 10.1080/19490976.2024.2395099. Epub 2024 Sep 6. Gut Microbes. 2024. PMID: 39239828 Free PMC article. Review.
References
-
- Arentsen T, Khalid R, Qian Y, and Diaz Heijtz R (2018). Sex-dependent alterations in motor and anxiety-like behavior of aged bacterial peptidoglycan sensing molecule 2 knockout mice. Brain, behavior, and immunity 67, 345–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

