Chloromethyl Glycosides as Versatile Synthons to Prepare Glycosyloxymethyl-Prodrugs
- PMID: 35045197
- PMCID: PMC9304170
- DOI: 10.1002/chem.202103910
Chloromethyl Glycosides as Versatile Synthons to Prepare Glycosyloxymethyl-Prodrugs
Abstract
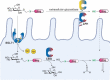
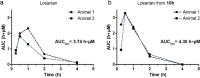
This work investigates the addition of monosaccharides to marketed drugs to improve their pharmacokinetic properties for oral absorption. To this end, a set of chloromethyl glycoside synthons were developed to prepare a variety of glycosyloxymethyl-prodrugs derived from 5-fluorouracil, thioguanine, propofol and losartan. Drug release was studied in vitro using β-glucosidase confirming rapid conversion of the monosaccharide prodrugs to release the parent drug, formaldehyde and the monosaccharide. To showcase this prodrug approach, a glucosyloxymethyl conjugate of the tetrazole-containing drug losartan was used for in vivo experiments and showed complete release of the drug in a dog-model.
Keywords: bioavailability; carbohydrates; glucose; glycosidase e; prodrugs.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Figures

Similar articles
-
Cytosolic beta-glycosidases for activation of glycoside prodrugs of daunorubicin.Biochem Pharmacol. 2003 Jun 1;65(11):1875-81. doi: 10.1016/s0006-2952(03)00183-7. Biochem Pharmacol. 2003. PMID: 12781339
-
Drug glycosides: potential prodrugs for colon-specific drug delivery.J Med Chem. 1985 Jan;28(1):51-7. doi: 10.1021/jm00379a012. J Med Chem. 1985. PMID: 3965714
-
Kinetic characterization of glycosidase activity from disaccharide conjugate to monosaccharide conjugate in Caco-2 cells.J Pharm Pharmacol. 2005 May;57(5):661-4. doi: 10.1211/0022357055948. J Pharm Pharmacol. 2005. PMID: 15901356
-
Prodrug design to improve pharmacokinetic and drug delivery properties: challenges to the discovery scientists.Curr Med Chem. 2010;17(32):3874-908. doi: 10.2174/092986710793205426. Curr Med Chem. 2010. PMID: 20858214 Review.
-
The use of esters as prodrugs for oral delivery of beta-lactam antibiotics.Pharm Biotechnol. 1998;11:345-65. doi: 10.1007/0-306-47384-4_15. Pharm Biotechnol. 1998. PMID: 9760687 Review.
References
-
- None
-
- Di L., Fish P. V., Mano T., Drug Discovery Today 2012, 17, 486–495; - PubMed
-
- Williams H. D., Trevaskis N. L., Charman S. A., Shanker R. M., Charman W. N., Pouton C. W., Porter C. J. H., Pharmacol. Rev. 2013, 65, 315–499. - PubMed
-
- None
-
- Rautio J., Meanwell N. A., Di L., Hageman M. J., Nat. Rev. Drug Discovery 2018, 17, 559–587; - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

