The ingenol-based protein kinase C agonist GSK445A is a potent inducer of HIV and SIV RNA transcription
- PMID: 35041707
- PMCID: PMC8797195
- DOI: 10.1371/journal.ppat.1010245
The ingenol-based protein kinase C agonist GSK445A is a potent inducer of HIV and SIV RNA transcription
Abstract
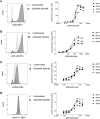
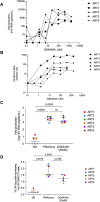
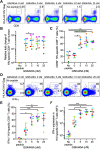
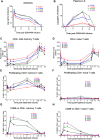
Activation of the NF-κB signaling pathway by Protein Kinase C (PKC) agonists is a potent mechanism for human immunodeficiency virus (HIV) latency disruption in vitro. However, significant toxicity risks and the lack of evidence supporting their activity in vivo have limited further evaluation of PKC agonists as HIV latency-reversing agents (LRA) in cure strategies. Here we evaluated whether GSK445A, a stabilized ingenol-B derivative, can induce HIV/simian immunodeficiency virus (SIV) transcription and virus production in vitro and demonstrate pharmacological activity in nonhuman primates (NHP). CD4+ T cells from people living with HIV and from SIV+ rhesus macaques (RM) on antiretroviral therapy (ART) exposed in vitro to 25 nM of GSK445A produced cell-associated viral transcripts as well as viral particles at levels similar to those induced by PMA/Ionomycin, indicating that GSK445A can potently reverse HIV/SIV latency. Importantly, these concentrations of GSK445A did not impair the proliferation or survival of HIV-specific CD8+ T cells, but instead, increased their numbers and enhanced IFN-γ production in response to HIV peptides. In vivo, GSK445A tolerability was established in SIV-naïve RM at 15 μg/kg although tolerability was reduced in SIV-infected RM on ART. Increases in plasma viremia following GSK445A administration were suggestive of increased SIV transcription in vivo. Collectively, these results indicate that GSK445A is a potent HIV/SIV LRA in vitro and has a tolerable safety profile amenable for further evaluation in vivo in NHP models of HIV cure/remission.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests. Jessica H. Brehm, Vincent Tai, Jun Tang and David Favre were employees of GlaxoSmithKline as the time when this work was performed. All other authors have declared that no competing interests exist.
Figures

Similar articles
-
Altered Response Pattern following AZD5582 Treatment of SIV-Infected, ART-Suppressed Rhesus Macaque Infants.J Virol. 2022 Apr 13;96(7):e0169921. doi: 10.1128/jvi.01699-21. Epub 2022 Mar 16. J Virol. 2022. PMID: 35293766 Free PMC article.
-
Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir.mBio. 2017 Aug 15;8(4):e01186-17. doi: 10.1128/mBio.01186-17. mBio. 2017. PMID: 28811349 Free PMC article.
-
In vitro effects of the small-molecule protein kinase C agonists on HIV latency reactivation.Sci Rep. 2016 Dec 12;6:39032. doi: 10.1038/srep39032. Sci Rep. 2016. PMID: 27941949 Free PMC article.
-
In Vitro and In Vivo Models of HIV Latency.Adv Exp Med Biol. 2018;1075:241-263. doi: 10.1007/978-981-13-0484-2_10. Adv Exp Med Biol. 2018. PMID: 30030796 Review.
-
Mechanisms of CD8+ T cell-mediated suppression of HIV/SIV replication.Eur J Immunol. 2018 Jun;48(6):898-914. doi: 10.1002/eji.201747172. Epub 2018 Mar 26. Eur J Immunol. 2018. PMID: 29427516 Free PMC article. Review.
Cited by
-
Potent latency reversal by Tat RNA-containing nanoparticle enables multi-omic analysis of the HIV-1 reservoir.Nat Commun. 2023 Dec 18;14(1):8397. doi: 10.1038/s41467-023-44020-5. Nat Commun. 2023. PMID: 38110433 Free PMC article.
-
Combined noncanonical NF-κB agonism and targeted BET bromodomain inhibition reverse HIV latency ex vivo.J Clin Invest. 2022 Apr 15;132(8):e157281. doi: 10.1172/JCI157281. J Clin Invest. 2022. PMID: 35426377 Free PMC article.
-
Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides.Molecules. 2022 Apr 21;27(9):2675. doi: 10.3390/molecules27092675. Molecules. 2022. PMID: 35566025 Free PMC article. Review.
-
Off-Target Effect of Activation of NF-κB by HIV Latency Reversal Agents on Transposable Elements Expression.Viruses. 2022 Jul 20;14(7):1571. doi: 10.3390/v14071571. Viruses. 2022. PMID: 35891551 Free PMC article.
-
Breaking the Silence: Regulation of HIV Transcription and Latency on the Road to a Cure.Viruses. 2023 Dec 15;15(12):2435. doi: 10.3390/v15122435. Viruses. 2023. PMID: 38140676 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

