Abiraterone switches castration-resistant prostate cancer dependency from adrenal androgens towards androgen receptor variants and glucocorticoid receptor signalling
- PMID: 35037287
- PMCID: PMC9306678
- DOI: 10.1002/pros.24297
Abiraterone switches castration-resistant prostate cancer dependency from adrenal androgens towards androgen receptor variants and glucocorticoid receptor signalling
Abstract
Introduction: Castration-resistant prostate cancer (CRPC) remains dependent on androgen receptor (AR) signalling, which is largely driven by conversion of adrenal androgen precursors lasting after castration. Abiraterone, an inhibitor of the steroidogenic enzyme CYP17A1, has been demonstrated to reduce adrenal androgen synthesis and prolong CRPC patient survival. To study mechanisms of resistance to castration and abiraterone, we created coculture models using human prostate and adrenal tumours.
Materials and methods: Castration-naïve and CRPC clones of VCaP were incubated with steroid substrates or cocultured with human adrenal cells (H295R) and treated with abiraterone or the antiandrogen enzalutamide. Male mice bearing VCaP xenografts with and without concurrent H295R xenografts were castrated and treated with placebo or abiraterone. Response was assessed by tumour growth and PSA release. Plasma and tumour steroid levels were assessed by LC/MS-MS. Quantitative polymerase chain reaction determined steroidogenic enzyme, nuclear receptor and AR target gene expression.
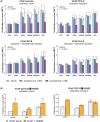
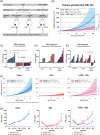
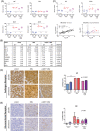
Results: In vitro, adrenal androgens induced castration-naïve and CRPC cell growth, while precursors steroids for de novo synthesis did not. In a coculture system, abiraterone blocked H295R-induced growth of VCaP cells. In vivo, H295R promoted castration-resistant VCaP growth. Abiraterone only inhibited VCaP growth or PSA production in the presence of H295R. Plasma steroid levels demonstrated CYP17A1 inhibition by abiraterone, whilst CRPC tumour tissue steroid levels showed no evidence of de novo intratumoural androgen production. Castration-resistant and abiraterone-resistant VCaP tumours had increased levels of AR, AR variants and glucocorticoid receptor (GR) resulting in equal AR target gene expression levels compared to noncastrate tumours.
Conclusions: In our model, ligand-dependent AR-regulated regrowth of CRPC was predominantly supported via adrenal androgen precursor production while there was no evidence for intratumoural androgen synthesis. Abiraterone-resistant tumours relied on AR overexpression, expression of ligand-independent AR variants and GR signalling.
Keywords: AR variants; CYP17A1 inhibition; abiraterone; adrenal androgens; androgen synthesis; castration resistant prostate cancer; glucocorticoid receptor; xenograft.
© 2022 The Authors. The Prostate published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

Similar articles
-
A bypass mechanism of abiraterone-resistant prostate cancer: Accumulating CYP17A1 substrates activate androgen receptor signaling.Prostate. 2019 Jun;79(9):937-948. doi: 10.1002/pros.23799. Epub 2019 Apr 24. Prostate. 2019. PMID: 31017696 Free PMC article.
-
Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants.Clin Cancer Res. 2011 Sep 15;17(18):5913-25. doi: 10.1158/1078-0432.CCR-11-0728. Epub 2011 Aug 1. Clin Cancer Res. 2011. PMID: 21807635 Free PMC article.
-
Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors.Cancer Res. 2011 Oct 15;71(20):6503-13. doi: 10.1158/0008-5472.CAN-11-0532. Epub 2011 Aug 25. Cancer Res. 2011. PMID: 21868758 Free PMC article.
-
Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis.Oncogene. 2014 May 29;33(22):2815-25. doi: 10.1038/onc.2013.235. Epub 2013 Jun 10. Oncogene. 2014. PMID: 23752196 Free PMC article. Review.
-
Mechanisms of drug resistance that target the androgen axis in castration resistant prostate cancer (CRPC).J Steroid Biochem Mol Biol. 2015 Sep;153:105-13. doi: 10.1016/j.jsbmb.2015.05.010. Epub 2015 May 29. J Steroid Biochem Mol Biol. 2015. PMID: 26032458 Free PMC article. Review.
Cited by
-
The future of patient-derived xenografts in prostate cancer research.Nat Rev Urol. 2023 Jun;20(6):371-384. doi: 10.1038/s41585-022-00706-x. Epub 2023 Jan 17. Nat Rev Urol. 2023. PMID: 36650259 Free PMC article. Review.
-
The role of 11-oxygenated androgens in prostate cancer.Endocr Oncol. 2023 Mar 13;3(1):e220072. doi: 10.1530/EO-22-0072. eCollection 2023 Jan 1. Endocr Oncol. 2023. PMID: 37434644 Free PMC article. Review.
-
Cell Line Characteristics Predict Subsequent Resistance to Androgen Receptor-Targeted Agents (ARTA) in Preclinical Models of Prostate Cancer.Front Oncol. 2022 Jun 13;12:877613. doi: 10.3389/fonc.2022.877613. eCollection 2022. Front Oncol. 2022. PMID: 35769712 Free PMC article.
-
Glucocorticoid Receptor Regulates and Interacts with LEDGF/p75 to Promote Docetaxel Resistance in Prostate Cancer Cells.Cells. 2023 Aug 11;12(16):2046. doi: 10.3390/cells12162046. Cells. 2023. PMID: 37626856 Free PMC article.
-
Glucocorticoid Receptor and β-Catenin Interact in Prostate Cancer Cells and Their Co-Inhibition Attenuates Tumorsphere Formation, Stemness, and Docetaxel Resistance.Int J Mol Sci. 2023 Apr 12;24(8):7130. doi: 10.3390/ijms24087130. Int J Mol Sci. 2023. PMID: 37108293 Free PMC article.
References
-
- Hofland J, van Weerden WM, Dits NF, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70(3):1256‐1264. - PubMed
-
- Luu‐The V, Belanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22(2):207‐221. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

