Translating genomic exploration of the family Polyomaviridae into confident human polyomavirus detection
- PMID: 35036862
- PMCID: PMC8749223
- DOI: 10.1016/j.isci.2021.103613
Translating genomic exploration of the family Polyomaviridae into confident human polyomavirus detection
Abstract
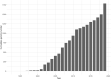
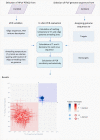
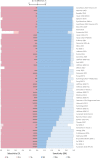
The Polyomaviridae is a family of ubiquitous dsDNA viruses that establish persistent infection early in life. Screening for human polyomaviruses (HPyVs), which comprise 14 diverse species, relies upon species-specific qPCRs whose validity may be challenged by accelerating genomic exploration of the virosphere. Using this reasoning, we tested 64 published HPyV qPCR assays in silico against the 1781 PyV genome sequences that were divided in targets and nontargets, based on anticipated species specificity of each qPCR. We identified several cases of problematic qPCR performance that were confirmed in vitro and corrected through using degenerate oligos. Furthermore, our study ranked 8 out of 52 tested BKPyV qPCRs as remaining of consistently high quality in the wake of recent PyV discoveries and showed how sensitivity of most other qPCRs could be rescued by annealing temperature adjustment. This study establishes an efficient framework for ensuring confidence in available HPyV qPCRs in the genomic era.
Keywords: Omics; Virology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
[New, newer, newest human polyomaviruses: how far?].Mikrobiyol Bul. 2013 Apr;47(2):362-81. doi: 10.5578/mb.5377. Mikrobiyol Bul. 2013. PMID: 23621738 Review. Turkish.
-
Multiplex detection in tonsillar tissue of all known human polyomaviruses.BMC Infect Dis. 2017 Jun 8;17(1):409. doi: 10.1186/s12879-017-2479-5. BMC Infect Dis. 2017. PMID: 28595595 Free PMC article.
-
Development and Evaluation of a Broad Bead-Based Multiplex Immunoassay To Measure IgG Seroreactivity against Human Polyomaviruses.J Clin Microbiol. 2018 Mar 26;56(4):e01566-17. doi: 10.1128/JCM.01566-17. Print 2018 Apr. J Clin Microbiol. 2018. PMID: 29305551 Free PMC article.
-
Role of BK human polyomavirus in cancer.Infect Agent Cancer. 2018 Apr 5;13:12. doi: 10.1186/s13027-018-0182-9. eCollection 2018. Infect Agent Cancer. 2018. PMID: 29632550 Free PMC article. Review.
-
Prevalence of DNA of fourteen human polyomaviruses determined in blood donors.Transfusion. 2019 Dec;59(12):3689-3697. doi: 10.1111/trf.15557. Epub 2019 Oct 21. Transfusion. 2019. PMID: 31633816 Free PMC article.
Cited by
-
Serology Identifies LIPyV as a Feline Rather than a Human Polyomavirus.Viruses. 2023 Jul 13;15(7):1546. doi: 10.3390/v15071546. Viruses. 2023. PMID: 37515232 Free PMC article.
-
Matrix Matters: Assessment of Commutability among BK Virus Assays and Standards.J Clin Microbiol. 2022 Sep 21;60(9):e0055522. doi: 10.1128/jcm.00555-22. Epub 2022 Aug 23. J Clin Microbiol. 2022. PMID: 35997500 Free PMC article.
References
-
- Bárcena-Panero A., Echevarría J.E., Romero-Gómez M.P., Royuela E., Castellanos A., González I., Fedele G. Development and validation with clinical samples of internally controlled multiplex real-time PCR for diagnosis of BKV and JCV infection in associated pathologies. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:173–179. doi: 10.1016/j.cimid.2011.12.010. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

