Coenzyme Q0 Inhibits NLRP3 Inflammasome Activation through Mitophagy Induction in LPS/ATP-Stimulated Macrophages
- PMID: 35035661
- PMCID: PMC8759827
- DOI: 10.1155/2022/4266214
Coenzyme Q0 Inhibits NLRP3 Inflammasome Activation through Mitophagy Induction in LPS/ATP-Stimulated Macrophages
Abstract
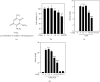
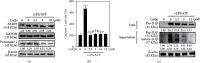
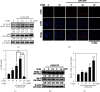
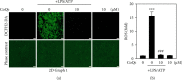
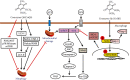
Coenzyme Q (CoQ) analogs with a variable number of isoprenoid units have exhibited as anti-inflammatory as well as antioxidant molecules. Using novel quinone derivative CoQ0 (2,3-dimethoxy-5-methyl-1,4-benzoquinone, zero side chain isoprenoid), we studied its molecular activities against LPS/ATP-induced inflammation and redox imbalance in murine RAW264.7 macrophages. CoQ0's non- or subcytotoxic concentration suppressed the NLRP3 inflammasome and procaspase-1 activation, followed by downregulation of IL1β expression in LPS/ATP-stimulated RAW264.7 macrophages. Similarly, treatment of CoQ0 led to LC3-I/II accumulation and p62/SQSTM1 activation. An increase in the Beclin-1/Bcl-2 ratio and a decrease in the expression of phosphorylated PI3K/AKT, p70 S6 kinase, and mTOR showed that autophagy was activated. Besides, CoQ0 increased Parkin protein to recruit damaged mitochondria and induced mitophagy in LPS/ATP-stimulated RAW264.7 macrophages. CoQ0 inhibited LPS/ATP-stimulated ROS generation in RAW264.7 macrophages. Notably, when LPS/ATP-stimulated RAW264.7 macrophages were treated with CoQ0, Mito-TEMPO (a mitochondrial ROS inhibitor), or N-acetylcysteine (NAC, a ROS inhibitor), there was a significant reduction of LPS/ATP-stimulated NLRP3 inflammasome activation and IL1β expression. Interestingly, treatment with CoQ0 or Mito-TEMPO, but not NAC, significantly increased LPS/ATP-induced LC3-II accumulation indicating that mitophagy plays a key role in the regulation of CoQ0-inhibited NLRP3 inflammasome activation. Nrf2 knockdown significantly decreased IL1β expression in LPS/ATP-stimulated RAW264.7 macrophages suggesting that CoQ0 inhibited ROS-mediated NLRP3 inflammasome activation and IL1β expression was suppressed due to the Nrf2 activation. Hence, this study showed that CoQ0 might be a promising candidate for the therapeutics of inflammatory disorders due to its effective anti-inflammatory as well as antioxidant properties.
Copyright © 2022 You-Cheng Hseu et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Coenzyme Q0 regulates NFκB/AP-1 activation and enhances Nrf2 stabilization in attenuation of LPS-induced inflammation and redox imbalance: Evidence from in vitro and in vivo studies.Biochim Biophys Acta. 2016 Feb;1859(2):246-61. doi: 10.1016/j.bbagrm.2015.11.001. Epub 2015 Nov 5. Biochim Biophys Acta. 2016. PMID: 26548719
-
Coenzyme Q0 defeats NLRP3-mediated inflammation, EMT/metastasis, and Warburg effects by inhibiting HIF-1α expression in human triple-negative breast cancer cells.Arch Toxicol. 2023 Apr;97(4):1047-1068. doi: 10.1007/s00204-023-03456-w. Epub 2023 Feb 27. Arch Toxicol. 2023. PMID: 36847822
-
FOXO3a acetylation regulates PINK1, mitophagy, inflammasome activation in murine palmitate-conditioned and diabetic macrophages.J Leukoc Biol. 2022 Mar;111(3):611-627. doi: 10.1002/JLB.3A0620-348RR. Epub 2021 Jul 20. J Leukoc Biol. 2022. PMID: 34288093
-
Polygala saponins inhibit NLRP3 inflammasome-mediated neuroinflammation via SHP-2-Mediated mitophagy.Free Radic Biol Med. 2022 Feb 1;179:76-94. doi: 10.1016/j.freeradbiomed.2021.12.263. Epub 2021 Dec 20. Free Radic Biol Med. 2022. PMID: 34933095 Review.
-
Mitochondrial dysfunction as a driver of NLRP3 inflammasome activation and its modulation through mitophagy for potential therapeutics.Int J Biochem Cell Biol. 2021 Jul;136:106013. doi: 10.1016/j.biocel.2021.106013. Epub 2021 May 19. Int J Biochem Cell Biol. 2021. PMID: 34022434 Review.
Cited by
-
The Role of Pyroptosis and Autophagy in the Nervous System.Mol Neurobiol. 2024 Mar;61(3):1271-1281. doi: 10.1007/s12035-023-03614-2. Epub 2023 Sep 12. Mol Neurobiol. 2024. PMID: 37697221 Free PMC article. Review.
-
Tolerable treatment of glioblastoma with redox-cycling 'mitocans': a comparative study in vivo.Redox Rep. 2023 Dec;28(1):2220531. doi: 10.1080/13510002.2023.2220531. Redox Rep. 2023. PMID: 37581329 Free PMC article.
-
Microbe-Derived Antioxidants Reduce Lipopolysaccharide-Induced Inflammatory Responses by Activating the Nrf2 Pathway to Inhibit the ROS/NLRP3/IL-1β Signaling Pathway.Int J Mol Sci. 2022 Oct 18;23(20):12477. doi: 10.3390/ijms232012477. Int J Mol Sci. 2022. PMID: 36293333 Free PMC article.
-
Research progress of mitochondrial dysfunction induced pyroptosis in acute lung injury.Respir Res. 2024 Nov 7;25(1):398. doi: 10.1186/s12931-024-03028-1. Respir Res. 2024. PMID: 39511593 Free PMC article. Review.
-
Mitochondria: An Emerging Unavoidable Link in the Pathogenesis of Periodontitis Caused by Porphyromonas gingivalis.Int J Mol Sci. 2024 Jan 6;25(2):737. doi: 10.3390/ijms25020737. Int J Mol Sci. 2024. PMID: 38255811 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

