Validation of a Saliva-Based Test for the Molecular Diagnosis of SARS-CoV-2 Infection
- PMID: 35035611
- PMCID: PMC8759915
- DOI: 10.1155/2022/6478434
Validation of a Saliva-Based Test for the Molecular Diagnosis of SARS-CoV-2 Infection
Abstract
Background: Since the beginning of the pandemic, clinicians and researchers have been searching for alternative tests to improve the screening and diagnosis of the SARS-CoV-2 infection. Currently, the gold standard for virus identification is the nasopharyngeal (NP) swab. Saliva samples, however, offer clear, practical, and logistical advantages but due to a lack of collection, transport, and storage solutions, high-throughput saliva-based laboratory tests are difficult to scale up as a screening or diagnostic tool. With this study, we aimed to validate an intralaboratory molecular detection method for SARS-CoV-2 on saliva samples collected in a new storage saline solution, comparing the results to NP swabs to determine the difference in sensitivity between the two tests.
Methods: In this study, 156 patients (cases) and 1005 asymptomatic subjects (controls) were enrolled and tested simultaneously for the detection of the SARS-CoV-2 viral genome by RT-PCR on both NP swab and saliva samples. Saliva samples were collected in a preservative and inhibiting saline solution (Biofarma Srl). Internal method validation was performed to standardize the entire workflow for saliva samples.
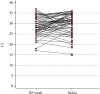
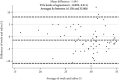
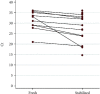
Results: The identification of SARS-CoV-2 conducted on saliva samples showed a clinical sensitivity of 95.1% and specificity of 97.8% compared to NP swabs. The positive predictive value (PPV) was 81% while the negative predictive value (NPV) was 99.5%. Test concordance was 97.6% (Cohen's Kappa = 0.86; 95% CI 0.81-0.91). The LoD of the test was 5 viral copies for both samples.
Conclusions: RT-PCR assays conducted on a stored saliva sample achieved similar performance to those on NP swabs, and this may provide a very effective tool for population screening and diagnosis. Collection of saliva in a stabilizing solution makes the test more convenient and widely available; furthermore, the denaturing properties of the solution reduce the infective risks belonging to sample manipulation.
Copyright © 2022 Michela Bulfoni et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Saliva is Comparable to Nasopharyngeal Swabs for Molecular Detection of SARS-CoV-2.Microbiol Spectr. 2021 Sep 3;9(1):e0016221. doi: 10.1128/Spectrum.00162-21. Epub 2021 Aug 18. Microbiol Spectr. 2021. PMID: 34406838 Free PMC article.
-
Comparison of SARS-CoV-2 PCR-Based Detection Using Saliva or Nasopharyngeal Swab Specimens in Asymptomatic Populations.Microbiol Spectr. 2021 Sep 3;9(1):e0006221. doi: 10.1128/Spectrum.00062-21. Epub 2021 Aug 25. Microbiol Spectr. 2021. PMID: 34431689 Free PMC article.
-
A Multiplex One-Step RT-qPCR Protocol to Detect SARS-CoV-2 in NP/OP Swabs and Saliva.Curr Protoc. 2021 May;1(5):e145. doi: 10.1002/cpz1.145. Curr Protoc. 2021. PMID: 34004070 Free PMC article.
-
Performance of Saliva, Oropharyngeal Swabs, and Nasal Swabs for SARS-CoV-2 Molecular Detection: a Systematic Review and Meta-analysis.J Clin Microbiol. 2021 Apr 20;59(5):e02881-20. doi: 10.1128/JCM.02881-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33504593 Free PMC article. Review.
-
Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature.Ear Nose Throat J. 2021 Apr;100(2_suppl):131S-138S. doi: 10.1177/0145561320953231. Epub 2020 Aug 31. Ear Nose Throat J. 2021. PMID: 32865458 Free PMC article. Review.
Cited by
-
Acceptability and Feasibility of Saliva-delivered PCR Coronavirus 2019 Tests for Young Children.Pediatrics. 2023 Jul 1;152(Suppl 1):e2022060352D. doi: 10.1542/peds.2022-060352D. Pediatrics. 2023. PMID: 37394507 Free PMC article.
-
Recent advances in RNA sample preparation techniques for the detection of SARS-CoV-2 in saliva and gargle.Trends Analyt Chem. 2023 Aug;165:117107. doi: 10.1016/j.trac.2023.117107. Epub 2023 May 23. Trends Analyt Chem. 2023. PMID: 37317683 Free PMC article. Review.
-
Accuracy of saliva for SARS-CoV-2 detection in outpatients and their household contacts during the circulation of the Omicron variant of concern.BMC Infect Dis. 2023 May 5;23(1):295. doi: 10.1186/s12879-023-08271-3. BMC Infect Dis. 2023. PMID: 37147601 Free PMC article.
-
Taking a step back from testing: Preanalytical considerations in molecular infectious disease diagnostics.Clin Biochem. 2023 May;115:22-32. doi: 10.1016/j.clinbiochem.2022.12.003. Epub 2022 Dec 8. Clin Biochem. 2023. PMID: 36495954 Free PMC article. Review.
-
Validation of RT-qPCR test for SARS-CoV-2 in saliva specimens.J Infect Public Health. 2022 Dec;15(12):1403-1408. doi: 10.1016/j.jiph.2022.10.028. Epub 2022 Nov 2. J Infect Public Health. 2022. PMID: 36371937 Free PMC article.
References
-
- CDC. Coronavirus Disease 2019 (COVID-19) USA: Centers for Disease Control and Prevention; 2020.
-
- Bhattacharya A. K., Maiti A. Coronavirus disease-2019: an overview and update. Bengal Physician Journal . 2019;6:45–47.
-
- Commissioner O of the. Which Uses a New Method of Saliva Sample Processing . FDA; 2020. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization to Yale School of Public Health for SalivaDirect.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

