Cortical tension initiates the positive feedback loop between cadherin and F-actin
- PMID: 35031276
- PMCID: PMC8874026
- DOI: 10.1016/j.bpj.2022.01.006
Cortical tension initiates the positive feedback loop between cadherin and F-actin
Abstract
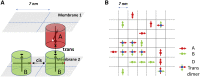
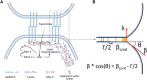
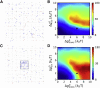
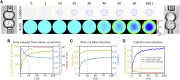
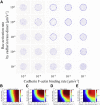
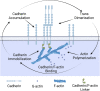
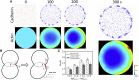
Adherens junctions physically link two cells at their contact interface via extracellular binding between cadherin molecules and intracellular interactions between cadherins and the actin cytoskeleton. Cadherin and actomyosin cytoskeletal dynamics are regulated reciprocally by mechanical and chemical signals, which subsequently determine the strength of cell-cell adhesions and the emergent organization and stiffness of the tissues they form. However, an understanding of the integrated system is lacking. We present a new mechanistic computational model of intercellular junction maturation in a cell doublet to investigate the mechanochemical cross talk that regulates adherens junction formation and homeostasis. The model couples a two-dimensional lattice-based simulation of cadherin dynamics with a reaction-diffusion representation of the reorganising actomyosin network through its regulation by Rho signalling at the intracellular junction. We demonstrate that local immobilization of cadherin induces cluster formation in a cis-less-dependent manner. We then recapitulate the process of cell-cell contact formation. Our model suggests that cortical tension applied on the contact rim can explain the ring distribution of cadherin and actin filaments (F-actin) on the cell-cell contact of the cell doublet. Furthermore, we propose and test the hypothesis that cadherin and F-actin interact like a positive feedback loop, which is necessary for formation of the ring structure. Different patterns of cadherin distribution were observed as an emergent property of disturbances of this positive feedback loop. We discuss these findings in light of available experimental observations on underlying mechanisms related to cadherin/F-actin binding and the mechanical environment.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Active tension: the role of cadherin adhesion and signaling in generating junctional contractility.Curr Top Dev Biol. 2015;112:65-102. doi: 10.1016/bs.ctdb.2014.11.016. Epub 2015 Feb 12. Curr Top Dev Biol. 2015. PMID: 25733138 Review.
-
Adhesion-induced cortical flows pattern E-cadherin-mediated cell contacts.Curr Biol. 2024 Jan 8;34(1):171-182.e8. doi: 10.1016/j.cub.2023.11.067. Epub 2023 Dec 21. Curr Biol. 2024. PMID: 38134934
-
Role of actin filaments and cis binding in cadherin clustering and patterning.PLoS Comput Biol. 2022 Jul 8;18(7):e1010257. doi: 10.1371/journal.pcbi.1010257. eCollection 2022 Jul. PLoS Comput Biol. 2022. PMID: 35802763 Free PMC article.
-
Actin-delimited adhesion-independent clustering of E-cadherin forms the nanoscale building blocks of adherens junctions.Dev Cell. 2015 Jan 26;32(2):139-54. doi: 10.1016/j.devcel.2014.12.003. Epub 2015 Jan 15. Dev Cell. 2015. PMID: 25600236
-
Integration of Cadherin Adhesion and Cytoskeleton at Adherens Junctions.Cold Spring Harb Perspect Biol. 2017 May 1;9(5):a028738. doi: 10.1101/cshperspect.a028738. Cold Spring Harb Perspect Biol. 2017. PMID: 28096263 Free PMC article. Review.
Cited by
-
Localization of cadherins in the postnatal cochlear epithelium and their relation to space formation.Dev Dyn. 2024 Aug;253(8):771-780. doi: 10.1002/dvdy.692. Epub 2024 Jan 24. Dev Dyn. 2024. PMID: 38264972
-
Nucleation of cadherin clusters on cell-cell interfaces.Sci Rep. 2022 Nov 2;12(1):18485. doi: 10.1038/s41598-022-23220-x. Sci Rep. 2022. PMID: 36323859 Free PMC article.
-
Actin polymerization and depolymerization in developing vertebrates.Front Physiol. 2023 Sep 8;14:1213668. doi: 10.3389/fphys.2023.1213668. eCollection 2023. Front Physiol. 2023. PMID: 37745245 Free PMC article. Review.
-
APC-driven actin nucleation powers collective cell dynamics in colorectal cancer cells.iScience. 2023 Apr 6;26(5):106583. doi: 10.1016/j.isci.2023.106583. eCollection 2023 May 19. iScience. 2023. PMID: 37128612 Free PMC article.
-
Different contractility modes control cell escape from multicellular spheroids and tumor explants.APL Bioeng. 2024 May 7;8(2):026110. doi: 10.1063/5.0188186. eCollection 2024 Jun. APL Bioeng. 2024. PMID: 38721268 Free PMC article.
References
-
- Sako Y., Nagafuchi A., et al. Kusumi A. Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: corralling and tethering by the membrane skeleton. J. Cell Biol. 1998;140:1227–1240. doi: 10.1083/jcb.140.5.1227. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

