The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic
- PMID: 35026151
- PMCID: PMC8702401
- DOI: 10.1016/j.cell.2021.12.032
The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic
Abstract
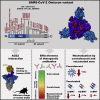
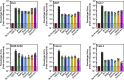
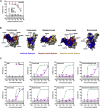
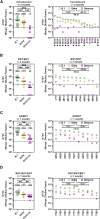
The rapid spread of the SARS-CoV-2 Omicron variant suggests that the virus might become globally dominant. Further, the high number of mutations in the viral spike protein raised concerns that the virus might evade antibodies induced by infection or vaccination. Here, we report that the Omicron spike was resistant against most therapeutic antibodies but remained susceptible to inhibition by sotrovimab. Similarly, the Omicron spike evaded neutralization by antibodies from convalescent patients or individuals vaccinated with the BioNTech-Pfizer vaccine (BNT162b2) with 12- to 44-fold higher efficiency than the spike of the Delta variant. Neutralization of the Omicron spike by antibodies induced upon heterologous ChAdOx1 (Astra Zeneca-Oxford)/BNT162b2 vaccination or vaccination with three doses of BNT162b2 was more efficient, but the Omicron spike still evaded neutralization more efficiently than the Delta spike. These findings indicate that most therapeutic antibodies will be ineffective against the Omicron variant and that double immunization with BNT162b2 might not adequately protect against severe disease induced by this variant.
Keywords: Omicron; SARS-CoV-2; antibody; immune evasion; neutralization; spike; vaccine.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
Omicron, the great escape artist.Nat Rev Immunol. 2022 Feb;22(2):75. doi: 10.1038/s41577-022-00676-6. Nat Rev Immunol. 2022. PMID: 35017722 Free PMC article.
-
Omicron's message on vaccines: Boosting begets breadth.Cell. 2022 Feb 3;185(3):411-413. doi: 10.1016/j.cell.2022.01.006. Epub 2022 Jan 14. Cell. 2022. PMID: 35065712 Free PMC article.
-
Beyond gut instincts: Microbe survival depends on sugars and butyrate.Cell. 2022 Feb 3;185(3):414-416. doi: 10.1016/j.cell.2021.12.037. Cell. 2022. PMID: 35120661
Similar articles
-
Characterization of Entry Pathways, Species-Specific Angiotensin-Converting Enzyme 2 Residues Determining Entry, and Antibody Neutralization Evasion of Omicron BA.1, BA.1.1, BA.2, and BA.3 Variants.J Virol. 2022 Sep 14;96(17):e0114022. doi: 10.1128/jvi.01140-22. Epub 2022 Aug 24. J Virol. 2022. PMID: 36000843 Free PMC article.
-
mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant.Cell Rep Med. 2022 Jan 24;3(2):100529. doi: 10.1016/j.xcrm.2022.100529. eCollection 2022 Feb 15. Cell Rep Med. 2022. PMID: 35233550 Free PMC article.
-
Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron.Nature. 2022 Feb;602(7898):682-688. doi: 10.1038/s41586-022-04399-5. Epub 2021 Dec 31. Nature. 2022. PMID: 35016197
-
Distinct Conformations of SARS-CoV-2 Omicron Spike Protein and Its Interaction with ACE2 and Antibody.Int J Mol Sci. 2023 Feb 14;24(4):3774. doi: 10.3390/ijms24043774. Int J Mol Sci. 2023. PMID: 36835186 Free PMC article. Review.
-
COVID-19: Structural Considerations for Virus Pathogenesis, Therapeutic Strategies and Vaccine Design in the Novel SARS-CoV-2 Variants Era.Mol Biotechnol. 2021 Oct;63(10):885-897. doi: 10.1007/s12033-021-00353-4. Epub 2021 Jun 18. Mol Biotechnol. 2021. PMID: 34145550 Free PMC article. Review.
Cited by
-
Humoral and Cellular Responses to a Delayed Fourth SARS-CoV-2 mRNA-Based Vaccine in Weak Responders to 3 Doses Kidney Transplant Recipients.Vaccines (Basel). 2022 Sep 1;10(9):1439. doi: 10.3390/vaccines10091439. Vaccines (Basel). 2022. PMID: 36146517 Free PMC article.
-
Medications for early treatment of COVID-19 in Australia.Med J Aust. 2022 Nov 6;217 Suppl 9(Suppl 9):S7-S13. doi: 10.5694/mja2.51750. Epub 2022 Oct 23. Med J Aust. 2022. PMID: 36273391 Free PMC article. Review.
-
Fluvoxamine for the treatment of COVID-19.Cochrane Database Syst Rev. 2022 Sep 14;9(9):CD015391. doi: 10.1002/14651858.CD015391. Cochrane Database Syst Rev. 2022. PMID: 36103313 Free PMC article. Review.
-
A single-dose MCMV-based vaccine elicits long-lasting immune protection in mice against distinct SARS-CoV-2 variants.Front Immunol. 2024 Jul 25;15:1383086. doi: 10.3389/fimmu.2024.1383086. eCollection 2024. Front Immunol. 2024. PMID: 39119342 Free PMC article.
-
Nasal irrigation efficiently attenuates SARS-CoV-2 Omicron infection, transmission and lung injury in the Syrian hamster model.iScience. 2022 Dec 22;25(12):105475. doi: 10.1016/j.isci.2022.105475. Epub 2022 Nov 2. iScience. 2022. PMID: 36338435 Free PMC article.
References
-
- Abdullah F. 2021. Tshwane District Omicron variant patient profile - early features.https://www.samrc.ac.za/news/tshwane-district-omicron-variant-patient-pr...
-
- Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.-L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58 doi: 10.1128/JCM.02107-20. - DOI - PMC - PubMed
-
- Arora P., Rocha C., Kempf A., Nehlmeier I., Graichen L., Winkler M.S., Lier M., Schulz S., Jäck H.-M., Cossmann A., et al. The spike protein of SARS-CoV-2 variant A.30 is heavily mutated and evades vaccine-induced antibodies with high efficiency. Cell. Mol. Immunol. 2021;18:2673–2675. doi: 10.1038/s41423-021-00779-5. - DOI - PMC - PubMed
-
- Arora P., Sidarovich A., Krüger N., Kempf A., Nehlmeier I., Graichen L., Moldenhauer A.-S., Winkler M.S., Schulz S., Jäck H.-M., et al. B.1.617.2 enters and fuses lung cells with increased efficiency and evades antibodies induced by infection and vaccination. Cell Rep. 2021;37:109825. doi: 10.1016/j.celrep.2021.109825. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

