Differential ER stress as a driver of cell fate following ricin toxin exposure
- PMID: 35024573
- PMCID: PMC8728110
- DOI: 10.1096/fba.2021-00005
Differential ER stress as a driver of cell fate following ricin toxin exposure
Abstract
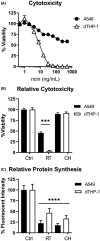
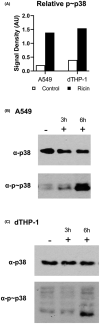
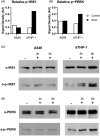
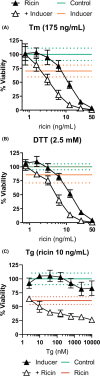
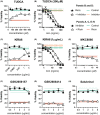
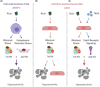
Inhalation of trace amounts of ricin toxin, a plant-derived ribosome-inactivating protein, results in ablation of alveolar macrophages, widespread epithelial damage, and the onset of acute respiratory distress syndrome (ARDS). While ricin's receptors are ubiquitous, certain cell types are more sensitive to ricin-induced cell death than others for reasons that remain unclear. For example, we demonstrate in side-by-side studies that macrophage-like differentiated THP-1 (dTHP-1) cells are hyper-sensitive to ricin, while lung epithelium-derived A549 cells are relatively insensitive, even though both cell types experience similar degrees of translational inhibition and p38 MAPK activation in response to ricin. Using a variety of small molecule inhibitors, we provide evidence that ER stress contributes to ricin-mediated cytotoxicity of dTHP-1 cells, but not A549 cells. On the other hand, the insensitivity of A549 cells to ricin was overcome by the addition of (TNF)-related apoptosis-inducing ligand (TRAIL; CD253), a known stimulator of extrinsic programmed cell death. These results have implications for understanding the complex pathophysiology of ricin-induced ARDS in that they demonstrate that intrinsic (e.g., ER stress) and extrinsic (e.g., TRAIL) factors may ultimately determine the fate of specific cell types following ricin intoxication.
Keywords: apoptosis; epithelium; inflammation; lung; macrophage; stress; toxin.
© 2021 The Authors. FASEB BioAdvances published by Wiley Periodicals LLC on behalf of The Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors have no financial or other competing interests to declare.
Figures


Similar articles
-
TRAIL (CD253) Sensitizes Human Airway Epithelial Cells to Toxin-Induced Cell Death.mSphere. 2018 Sep 26;3(5):e00399-18. doi: 10.1128/mSphere.00399-18. mSphere. 2018. PMID: 30258037 Free PMC article.
-
TNF Family Cytokines Induce Distinct Cell Death Modalities in the A549 Human Lung Epithelial Cell Line when Administered in Combination with Ricin Toxin.Toxins (Basel). 2019 Aug 1;11(8):450. doi: 10.3390/toxins11080450. Toxins (Basel). 2019. PMID: 31374990 Free PMC article.
-
Necroptosis of Lung Epithelial Cells Triggered by Ricin Toxin and Bystander Inflammation.Cell Physiol Biochem. 2023 Jan 25;57(1):1-14. doi: 10.33594/000000601. Cell Physiol Biochem. 2023. PMID: 36695077
-
The induction of apoptosis by Shiga toxins and ricin.Curr Top Microbiol Immunol. 2012;357:137-78. doi: 10.1007/82_2011_155. Curr Top Microbiol Immunol. 2012. PMID: 22130961 Review.
-
Intracellular Transport and Cytotoxicity of the Protein Toxin Ricin.Toxins (Basel). 2019 Jun 18;11(6):350. doi: 10.3390/toxins11060350. Toxins (Basel). 2019. PMID: 31216687 Free PMC article. Review.
Cited by
-
Gut ribotoxic stress responses facilitate dyslipidemia via metabolic reprogramming: an environmental health prediction.Theranostics. 2024 Jan 20;14(3):1289-1311. doi: 10.7150/thno.88586. eCollection 2024. Theranostics. 2024. PMID: 38323314 Free PMC article.
-
Induction of the PERK-eIF2α-ATF4 Pathway in M1 Macrophages under Endoplasmic Reticulum Stress.Dokl Biochem Biophys. 2024 Aug;517(1):264-268. doi: 10.1134/S1607672924600301. Epub 2024 Jul 13. Dokl Biochem Biophys. 2024. PMID: 39002013 Free PMC article.
References
-
- Sapoznikov A, Gal Y, Falach R, et al. Early disruption of the alveolar‐capillary barrier in a ricin‐induced ARDS mouse model: neutrophil‐dependent and ‐independent impairment of junction proteins. Am J Physiol Lung Cell Mol Physiol. 2019;316:L255‐L268. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
