Identification of polo-like kinase 1 as a therapeutic target in murine lupus
- PMID: 35024139
- PMCID: PMC8733964
- DOI: 10.1002/cti2.1362
Identification of polo-like kinase 1 as a therapeutic target in murine lupus
Abstract
Introduction: The signalling cascades that contribute to lupus pathogenesis are incompletely understood. We address this by using an unbiased activity-based kinome screen of murine lupus.
Methods: An unbiased activity-based kinome screen (ABKS) of 196 kinases was applied to two genetically different murine lupus strains. Systemic and renal lupus were evaluated following in vivo PLK1blockade. The upstream regulators and downstream targets of PLK1 were also interrogated.
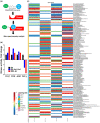
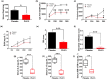
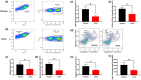
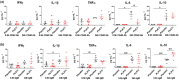
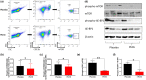
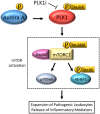
Results: Multiple signalling cascades were noted to be more active in murine lupus spleens, including PLK1. In vivo administration of a PLK1-specific inhibitor ameliorated splenomegaly, anti-dsDNA antibody production, proteinuria, BUN and renal pathology in MRL.lpr mice (P < 0.05). Serum IL-6, IL-17 and kidney injury molecule 1 (KIM-1) were significantly decreased after PLK1 inhibition. PLK1 inhibition reduced germinal centre and marginal zone B cells in the spleen, but changes in T cells were not significant. In vitro, splenocytes were treated with anti-mouse CD40 Ab or F(ab')2 fragment anti-mouse IgM. After 24-h stimulation, IL-6 secretion was significantly reduced upon PLK1 blockade, whereas IL-10 production was significantly increased. The phosphorylation of mTOR was assessed in splenocyte subsets, which revealed a significant change in myeloid cells. PLK1 blockade reduced phosphorylation associated with mTOR signalling, while Aurora-A emerged as a potential upstream regulator of PLK1.
Conclusion: The Aurora-A → PLK1 → mTOR signalling axis may be central in lupus pathogenesis, and emerges as a potential therapeutic target.
Keywords: PLK1; SLE; drug target; kinase activity.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
BTK inhibition ameliorates kidney disease in spontaneous lupus nephritis.Clin Immunol. 2018 Dec;197:205-218. doi: 10.1016/j.clim.2018.10.008. Epub 2018 Oct 16. Clin Immunol. 2018. PMID: 30339790 Free PMC article.
-
Interleukin-9 Is Associated with Elevated Anti-Double-Stranded DNA Antibodies in Lupus-Prone Mice.Mol Med. 2015 Apr 15;21(1):364-70. doi: 10.2119/molmed.2014.00237. Mol Med. 2015. PMID: 25902303 Free PMC article.
-
HM71224, a selective Bruton's tyrosine kinase inhibitor, attenuates the development of murine lupus.Arthritis Res Ther. 2017 Sep 26;19(1):211. doi: 10.1186/s13075-017-1402-1. Arthritis Res Ther. 2017. PMID: 28950886 Free PMC article.
-
Therapeutic effects of the artemisinin analog SM934 on lupus-prone MRL/lpr mice via inhibition of TLR-triggered B-cell activation and plasma cell formation.Cell Mol Immunol. 2016 May;13(3):379-90. doi: 10.1038/cmi.2015.13. Epub 2015 Mar 16. Cell Mol Immunol. 2016. PMID: 25942599 Free PMC article.
-
[Effect of CD40 siRNA on inflammatory response of MRL/Lpr mice].Beijing Da Xue Xue Bao Yi Xue Ban. 2016 Oct 18;48(5):771-776. Beijing Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27752154 Chinese.
Cited by
-
Multiple Roles of PLK1 in Mitosis and Meiosis.Cells. 2023 Jan 2;12(1):187. doi: 10.3390/cells12010187. Cells. 2023. PMID: 36611980 Free PMC article. Review.
References
-
- Lipsky PE. Systemic lupus erythematosus: an autoimmune disease of B cell hyperactivity. Nat Immunol 2001; 2: 764–766. - PubMed
-
- Morand EF, Mosca M. Treat to target, remission and low disease activity in SLE. Best Pract Res Clin Rheumatol 2017; 31: 342–350. - PubMed
-
- Cassia M, Alberici F, Gallieni M, Jayne D. Lupus nephritis and B‐cell targeting therapy. Expert Rev Clin Immunol 2017; 13: 951–962. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
