Amyloid-beta peptide and tau protein crosstalk in Alzheimer's disease
- PMID: 35017413
- PMCID: PMC8820696
- DOI: 10.4103/1673-5374.332127
Amyloid-beta peptide and tau protein crosstalk in Alzheimer's disease
Abstract
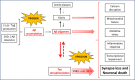
Alzheimer's disease is a neurodegenerative disease that accounts for most of the 50-million dementia cases worldwide in 2018. A large amount of evidence supports the amyloid cascade hypothesis, which states that amyloid-beta accumulation triggers tau hyperphosphorylation and aggregation in form of neurofibrillary tangles, and these aggregates lead to inflammation, synaptic impairment, neuronal loss, and thus to cognitive decline and behavioral abnormalities. The poor correlation found between cognitive decline and amyloid plaques, have led the scientific community to question whether amyloid-beta accumulation is actually triggering neurodegeneration in Alzheimer's disease. The occurrence of tau neurofibrillary tangles better correlates to neuronal loss and clinical symptoms and, although amyloid-beta may initiate the cascade of events, tau impairment is likely the effector molecule of neurodegeneration. Recently, it has been shown that amyloid-beta and tau cooperatively work to impair transcription of genes involved in synaptic function and, more importantly, that downregulation of tau partially reverses transcriptional perturbations. Despite mounting evidence points to an interplay between amyloid-beta and tau, some factors could independently affect both pathologies. Thus, the dual pathway hypothesis, which states that there are common upstream triggers causing both amyloid-beta and tau abnormalities has been proposed. Among others, the immune system seems to be strongly involved in amyloid-beta and tau pathologies. Other factors, as the apolipoprotein E ε4 isoform has been suggested to act as a link between amyloid-beta and tau hyperphosphorylation. Interestingly, amyloid-beta-immunotherapy reduces not only amyloid-beta but also tau levels in animal models and in clinical trials. Likewise, it has been shown that tau-immunotherapy also reduces amyloid-beta levels. Thus, even though amyloid-beta immunotherapy is more advanced than tau-immunotherapy, combined amyloid-beta and tau-directed therapies at early stages of the disease have recently been proposed as a strategy to stop the progression of Alzheimer's disease.
Keywords: Alzheimer; aggregation; amyloid-beta; dementia; immunotherapy; inflammation; neurodegeneration; tau.
Conflict of interest statement
Figures


Similar articles
-
A three-dimensional human neural cell culture model of Alzheimer's disease.Nature. 2014 Nov 13;515(7526):274-8. doi: 10.1038/nature13800. Epub 2014 Oct 12. Nature. 2014. PMID: 25307057 Free PMC article.
-
Amyloid-induced neurofibrillary tangle formation in Alzheimer's disease: insight from transgenic mouse and tissue-culture models.Int J Dev Neurosci. 2004 Nov;22(7):453-65. doi: 10.1016/j.ijdevneu.2004.07.013. Int J Dev Neurosci. 2004. PMID: 15465275 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
-
Immunotherapy for the treatment of Alzheimer's disease: amyloid-β or tau, which is the right target?Immunotargets Ther. 2013 Dec 27;3:19-28. doi: 10.2147/ITT.S40131. eCollection 2014. Immunotargets Ther. 2013. PMID: 27471697 Free PMC article. Review.
Cited by
-
Demand Coupling Drives Neurodegeneration: A Model of Age-Related Cognitive Decline and Dementia.Cells. 2022 Sep 7;11(18):2789. doi: 10.3390/cells11182789. Cells. 2022. PMID: 36139364 Free PMC article. Review.
-
Uncovering the Early Events Associated with Oligomeric Aβ-Induced Src Activation.Antioxidants (Basel). 2023 Sep 16;12(9):1770. doi: 10.3390/antiox12091770. Antioxidants (Basel). 2023. PMID: 37760073 Free PMC article.
-
Rbm8a regulates neurogenesis and reduces Alzheimer's disease-associated pathology in the dentate gyrus of 5×FAD mice.Neural Regen Res. 2024 Apr;19(4):863-871. doi: 10.4103/1673-5374.382254. Neural Regen Res. 2024. PMID: 37843222 Free PMC article.
-
Reperfusion after hypoxia-ischemia exacerbates brain injury with compensatory activation of the anti- ferroptosis system: based on a novel rat model.Neural Regen Res. 2023 Oct;18(10):2229-2236. doi: 10.4103/1673-5374.369117. Neural Regen Res. 2023. PMID: 37056142 Free PMC article.
-
Developments in scalable strategies for detecting early markers of cognitive decline.Transl Psychiatry. 2022 Nov 9;12(1):473. doi: 10.1038/s41398-022-02237-w. Transl Psychiatry. 2022. PMID: 36351888 Free PMC article. Review.
References
-
- Abushakra S, Porsteinsson A, Scheltens P, Sadowsky C, Vellas B, Cummings J, Gauthier S, Hey JA, Power A, Wang P, Shen L, Tolar M. Clinical effects of tramiprosate in APOE4/4 homozygous patients with mild Alzheimer’s disease suggest disease modification potential. J Prev Alzheimers Dis. 2017;4:149–156. - PubMed
-
- Alawieyah Syed Mortadza S, Sim JA, Neubrand VE, Jiang LH. A critical role of TRPM2 channel in Aβ42-induced microglial activation and generation of tumor necrosis factor-α. Glia. 2018;66:562–575. - PubMed
-
- Alzforum. AAT-AD/PDTM 2020 Conference: Advances in Alzheimer’s and Parkinson’s Therapies. 2020
-
- Alzheimer’s Disease International. World Alzheimer Report 2018 -The state of the art of dementia research: New frontiers. 2018
Publication types
LinkOut - more resources
Full Text Sources

