Neurobiological Mechanisms of Nicotine Reward and Aversion
- PMID: 35017179
- PMCID: PMC11060337
- DOI: 10.1124/pharmrev.121.000299
Neurobiological Mechanisms of Nicotine Reward and Aversion
Abstract
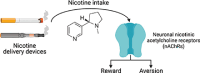
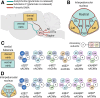
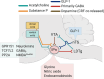
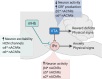
Neuronal nicotinic acetylcholine receptors (nAChRs) regulate the rewarding actions of nicotine contained in tobacco that establish and maintain the smoking habit. nAChRs also regulate the aversive properties of nicotine, sensitivity to which decreases tobacco use and protects against tobacco use disorder. These opposing behavioral actions of nicotine reflect nAChR expression in brain reward and aversion circuits. nAChRs containing α4 and β2 subunits are responsible for the high-affinity nicotine binding sites in the brain and are densely expressed by reward-relevant neurons, most notably dopaminergic, GABAergic, and glutamatergic neurons in the ventral tegmental area. High-affinity nAChRs can incorporate additional subunits, including β3, α6, or α5 subunits, with the resulting nAChR subtypes playing discrete and dissociable roles in the stimulatory actions of nicotine on brain dopamine transmission. nAChRs in brain dopamine circuits also participate in aversive reactions to nicotine and the negative affective state experienced during nicotine withdrawal. nAChRs containing α3 and β4 subunits are responsible for the low-affinity nicotine binding sites in the brain and are enriched in brain sites involved in aversion, including the medial habenula, interpeduncular nucleus, and nucleus of the solitary tract, brain sites in which α5 nAChR subunits are also expressed. These aversion-related brain sites regulate nicotine avoidance behaviors, and genetic variation that modifies the function of nAChRs in these sites increases vulnerability to tobacco dependence and smoking-related diseases. Here, we review the molecular, cellular, and circuit-level mechanisms through which nicotine elicits reward and aversion and the adaptations in these processes that drive the development of nicotine dependence. SIGNIFICANCE STATEMENT: Tobacco use disorder in the form of habitual cigarette smoking or regular use of other tobacco-related products is a major cause of death and disease worldwide. This article reviews the actions of nicotine in the brain that contribute to tobacco use disorder.
Copyright © 2022 The Author(s).
Figures
Similar articles
-
Addiction-related neuroadaptations following chronic nicotine exposure.J Neurochem. 2021 Jun;157(5):1652-1673. doi: 10.1111/jnc.15356. J Neurochem. 2021. PMID: 33742685 Review.
-
α3* Nicotinic Acetylcholine Receptors in the Habenula-Interpeduncular Nucleus Circuit Regulate Nicotine Intake.J Neurosci. 2021 Feb 24;41(8):1779-1787. doi: 10.1523/JNEUROSCI.0127-19.2020. Epub 2020 Dec 30. J Neurosci. 2021. PMID: 33380469 Free PMC article.
-
β4-Nicotinic Receptors Are Critically Involved in Reward-Related Behaviors and Self-Regulation of Nicotine Reinforcement.J Neurosci. 2020 Apr 22;40(17):3465-3477. doi: 10.1523/JNEUROSCI.0356-19.2020. Epub 2020 Mar 17. J Neurosci. 2020. PMID: 32184221 Free PMC article.
-
Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability.Neuropharmacology. 2014 Jan;76 Pt B(0 0):533-44. doi: 10.1016/j.neuropharm.2013.09.008. Epub 2013 Sep 18. Neuropharmacology. 2014. PMID: 24055497 Free PMC article. Review.
-
Molecular mechanisms underlying behaviors related to nicotine addiction.Cold Spring Harb Perspect Med. 2013 Jan 1;3(1):a012112. doi: 10.1101/cshperspect.a012112. Cold Spring Harb Perspect Med. 2013. PMID: 23143843 Free PMC article. Review.
Cited by
-
Society for Research on Nicotine and Tobacco as an Outgrowth of the 1988 Surgeon General's Report on Nicotine Addiction: Reflections of the Early Presidents.Nicotine Tob Res. 2024 Jan 22;26(2):118-125. doi: 10.1093/ntr/ntad151. Nicotine Tob Res. 2024. PMID: 37584666 Free PMC article. Review.
-
Expression pattern of nicotinic acetylcholine receptor subunit transcripts in neurons and astrocytes in the ventral tegmental area and locus coeruleus.Eur J Neurosci. 2024 May;59(9):2225-2239. doi: 10.1111/ejn.16109. Epub 2023 Aug 4. Eur J Neurosci. 2024. PMID: 37539749 Free PMC article.
-
Prolonged nicotine exposure reduces aversion to the drug in mice by altering nicotinic transmission in the interpeduncular nucleus.Elife. 2023 May 30;12:e80767. doi: 10.7554/eLife.80767. Elife. 2023. PMID: 37249215 Free PMC article.
-
Hedgehog-interacting protein acts in the habenula to regulate nicotine intake.Proc Natl Acad Sci U S A. 2022 Nov 15;119(46):e2209870119. doi: 10.1073/pnas.2209870119. Epub 2022 Nov 8. Proc Natl Acad Sci U S A. 2022. PMID: 36346845 Free PMC article.
-
A distributed auditory network mediated by pontine central gray underlies ultra-fast awakening in response to alerting sounds.Curr Biol. 2024 Oct 21;34(20):4597-4611.e5. doi: 10.1016/j.cub.2024.08.020. Epub 2024 Sep 11. Curr Biol. 2024. PMID: 39265569
References
-
- Acquas E, Carboni E, Leone P, Di Chiara G (1989) SCH 23390 blocks drug-conditioned place-preference and place-aversion: anhedonia (lack of reward) or apathy (lack of motivation) after dopamine-receptor blockade? Psychopharmacology (Berl) 99:151–155. - PubMed
-
- Agetsuma MAizawa HAoki T, et al. (2010) The habenula is crucial for experiencedependent modification of fear responses in zebrafish. Nat Neurosci 13:1354–1356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
