DSTYK Enhances Chemoresistance in Triple-Negative Breast Cancer Cells
- PMID: 35011659
- PMCID: PMC8750327
- DOI: 10.3390/cells11010097
DSTYK Enhances Chemoresistance in Triple-Negative Breast Cancer Cells
Abstract
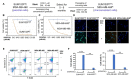
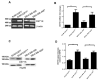
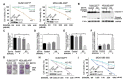
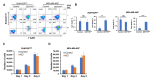
Breast cancer, as the most prevalent cancer in women, is responsible for more than 15% of new cancer cases and about 6.9% of all cancer-related death in the US. A major cause of therapeutic failure in breast cancer is the development of resistance to chemotherapy, especially for triple-negative breast cancer (TNBC). Therefore, how to overcome chemoresistance is the major challenge to improve the life expectancy of breast cancer patients. Our studies demonstrate that TNBC cells surviving the chronic treatment of chemotherapeutic drugs show significantly higher expression of the dual serine/threonine and tyrosine protein kinase (DSTYK) than non-treated parental cells. In our in vitro cellular models, DSTYK knockout via the CRISPR/Cas9-mediated technique results in apoptotic cell death of chemoresistant cells upon drug treatment. Moreover, DSTYK knockout promotes chemotherapeutic drug-induced tumor cell death in an orthotopic mouse model. These findings suggest that DSTYK exerts an important and previously unknown role in promoting chemoresistance. Our studies provide fundamental insight into the role of DSTYK in chemoresistance in TNBC cells and lay the foundation for the development of new strategies targeting DSTYK for improving TNBC therapy.
Keywords: CRISPR/Cas9; DSTYK; TNBC; breast cancer; chemoresistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
DSTYK Promotes Metastasis and Chemoresistance via EMT in Colorectal Cancer.Front Pharmacol. 2020 Sep 2;11:1250. doi: 10.3389/fphar.2020.01250. eCollection 2020. Front Pharmacol. 2020. PMID: 32982725 Free PMC article.
-
Inhibition of Notch1 reverses EMT and chemoresistance to cisplatin via direct downregulation of MCAM in triple-negative breast cancer cells.Int J Cancer. 2020 Jul 15;147(2):490-504. doi: 10.1002/ijc.32911. Epub 2020 Feb 15. Int J Cancer. 2020. Retraction in: Int J Cancer. 2024 Feb 1;154(3):E4. doi: 10.1002/ijc.34792 PMID: 32020593 Retracted.
-
4-Acetylantroquinonol B induced DNA damage response signaling and apoptosis via suppressing CDK2/CDK4 expression in triple negative breast cancer cells.Toxicol Appl Pharmacol. 2021 Jul 1;422:115493. doi: 10.1016/j.taap.2021.115493. Epub 2021 Mar 13. Toxicol Appl Pharmacol. 2021. PMID: 33727089
-
An insight into the cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2021 Nov;17(31):4185-4206. doi: 10.2217/fon-2021-0172. Epub 2021 Aug 3. Future Oncol. 2021. PMID: 34342489 Review.
-
Molecular targets and therapeutics in chemoresistance of triple-negative breast cancer.Med Oncol. 2021 Nov 23;39(1):14. doi: 10.1007/s12032-021-01610-x. Med Oncol. 2021. PMID: 34812991 Review.
Cited by
-
Whole-exome sequencing of Indian prostate cancer reveals a novel therapeutic target: POLQ.J Cancer Res Clin Oncol. 2023 Jun;149(6):2451-2462. doi: 10.1007/s00432-022-04111-0. Epub 2022 Jun 23. J Cancer Res Clin Oncol. 2023. PMID: 35737091
-
DSTYK inhibition increases the sensitivity of lung cancer cells to T cell-mediated cytotoxicity.J Exp Med. 2022 Dec 5;219(12):e20220726. doi: 10.1084/jem.20220726. Epub 2022 Sep 28. J Exp Med. 2022. PMID: 36169652 Free PMC article.
-
LncRNA MCM3AP-AS1 promotes chemoresistance in triple-negative breast cancer through the miR-524-5p/RBM39 axis.Mol Cell Biochem. 2025 Jan;480(1):371-384. doi: 10.1007/s11010-023-04908-8. Epub 2024 Mar 12. Mol Cell Biochem. 2025. PMID: 38472681
-
Nanomaterial-assisted CRISPR gene-engineering - A hallmark for triple-negative breast cancer therapeutics advancement.Mater Today Bio. 2022 Oct 4;16:100450. doi: 10.1016/j.mtbio.2022.100450. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36267139 Free PMC article. Review.
-
Targeting c-Met in breast cancer: From mechanisms of chemoresistance to novel therapeutic strategies.Curr Res Pharmacol Drug Discov. 2024 Oct 22;7:100204. doi: 10.1016/j.crphar.2024.100204. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39524211 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

