Folate Transport and One-Carbon Metabolism in Targeted Therapies of Epithelial Ovarian Cancer
- PMID: 35008360
- PMCID: PMC8750473
- DOI: 10.3390/cancers14010191
Folate Transport and One-Carbon Metabolism in Targeted Therapies of Epithelial Ovarian Cancer
Abstract
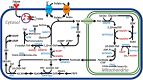
New therapies are urgently needed for epithelial ovarian cancer (EOC), the most lethal gynecologic malignancy. To identify new approaches for targeting EOC, metabolic vulnerabilities must be discovered and strategies for the selective delivery of therapeutic agents must be established. Folate receptor (FR) α and the proton-coupled folate transporter (PCFT) are expressed in the majority of EOCs. FRβ is expressed on tumor-associated macrophages, a major infiltrating immune population in EOC. One-carbon (C1) metabolism is partitioned between the cytosol and mitochondria and is important for the synthesis of nucleotides, amino acids, glutathione, and other critical metabolites. Novel inhibitors are being developed with the potential for therapeutic targeting of tumors via FRs and the PCFT, as well as for inhibiting C1 metabolism. In this review, we summarize these exciting new developments in targeted therapies for both tumors and the tumor microenvironment in EOC.
Keywords: epithelial ovarian cancer; folate; folate receptor; folate transport; one-carbon metabolism; proton-coupled folate transporter; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Targeted therapy of pyrrolo[2,3-d]pyrimidine antifolates in a syngeneic mouse model of high grade serous ovarian cancer and the impact on the tumor microenvironment.Sci Rep. 2022 Jul 5;12(1):11346. doi: 10.1038/s41598-022-14788-5. Sci Rep. 2022. PMID: 35790779 Free PMC article.
-
Folate-appended cyclodextrin carrier targets ovarian cancer cells expressing the proton-coupled folate transporter.Cancer Sci. 2020 May;111(5):1794-1804. doi: 10.1111/cas.14379. Epub 2020 Apr 3. Cancer Sci. 2020. PMID: 32154964 Free PMC article.
-
Dual Targeting of Epithelial Ovarian Cancer Via Folate Receptor α and the Proton-Coupled Folate Transporter with 6-Substituted Pyrrolo[2,3-d]pyrimidine Antifolates.Mol Cancer Ther. 2017 May;16(5):819-830. doi: 10.1158/1535-7163.MCT-16-0444. Epub 2017 Jan 30. Mol Cancer Ther. 2017. PMID: 28138029 Free PMC article.
-
Biology of the major facilitative folate transporters SLC19A1 and SLC46A1.Curr Top Membr. 2014;73:175-204. doi: 10.1016/B978-0-12-800223-0.00004-9. Curr Top Membr. 2014. PMID: 24745983 Free PMC article. Review.
-
Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications.Cancer Metastasis Rev. 2015 Mar;34(1):41-52. doi: 10.1007/s10555-014-9539-8. Cancer Metastasis Rev. 2015. PMID: 25564455 Review.
Cited by
-
Mitochondrial and Cytosolic One-Carbon Metabolism Is a Targetable Metabolic Vulnerability in Cisplatin-Resistant Ovarian Cancer.Mol Cancer Ther. 2024 Jun 4;23(6):809-822. doi: 10.1158/1535-7163.MCT-23-0550. Mol Cancer Ther. 2024. PMID: 38377173 Free PMC article.
-
Nomogram based on pretreatment hepatic and renal function indicators for survival prediction of locally advanced esophageal squamous cell carcinoma with treatment of neoadjuvant chemoradiotherapy plus surgery.Updates Surg. 2024 Aug;76(4):1377-1388. doi: 10.1007/s13304-023-01693-3. Epub 2023 Nov 13. Updates Surg. 2024. PMID: 37957531
-
Glutathione Transferase P1: Potential Therapeutic Target in Ovarian Cancer.Medicina (Kaunas). 2022 Nov 16;58(11):1660. doi: 10.3390/medicina58111660. Medicina (Kaunas). 2022. PMID: 36422199 Free PMC article. Review.
-
Serine-associated one-carbon metabolic reprogramming: a new anti-cancer therapeutic strategy.Front Oncol. 2023 Aug 18;13:1184626. doi: 10.3389/fonc.2023.1184626. eCollection 2023. Front Oncol. 2023. PMID: 37664062 Free PMC article. Review.
-
Advanced stage, high-grade primary tumor ovarian cancer: a multi-omics dissection and biomarker prediction process.Sci Rep. 2023 Oct 12;13(1):17265. doi: 10.1038/s41598-023-44246-9. Sci Rep. 2023. PMID: 37828118 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

