The Tumour Suppressor CYLD Is Required for Clathrin-Mediated Endocytosis of EGFR and Cetuximab-Induced Apoptosis in Head and Neck Squamous Cell Carcinoma
- PMID: 35008337
- PMCID: PMC8750287
- DOI: 10.3390/cancers14010173
The Tumour Suppressor CYLD Is Required for Clathrin-Mediated Endocytosis of EGFR and Cetuximab-Induced Apoptosis in Head and Neck Squamous Cell Carcinoma
Abstract
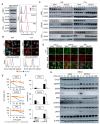
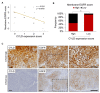
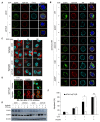
Epidermal growth factor receptor (EGFR) is frequently overexpressed in head and neck squamous cell carcinoma (HNSCC) and is a target for the therapeutic antibody cetuximab (CTX). However, because only some patients have a significant clinical response to CTX, identification of its predictive biomarkers and potentiation of CTX-based therapies are important. We have recently reported a frequent downregulation of cylindromatosis (CYLD) in primary HNSCC, which led to increased cell invasion and cisplatin resistance. Here, we show that CYLD located mainly in lipid rafts was required for clathrin-mediated endocytosis (CME) and degradation of the EGFR induced by EGF and CTX in HNSCC cells. The N-terminus containing the first cytoskeleton-associated protein-glycine domain of CYLD was responsible for this regulation. Loss of CYLD restricted EGFR to lipid rafts, which suppressed CTX-induced apoptosis without impeding CTX's inhibitory activity against downstream signalling pathways. Disruption of the lipid rafts with cholesterol-removing agents overcame this resistance by restoring CME and the degradation of EGFR. Regulation of EGFR trafficking by CYLD is thus critical for the antitumour activity of CTX. Our findings suggest the usefulness of a combination of cholesterol-lowering drugs with anti-EGFR antibody therapy in HNSCC.
Keywords: CYLD; EGFR; cetuximab; clathrin-mediated endocytosis; head and neck squamous cell carcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Novel EGFR ectodomain mutations associated with ligand-independent activation and cetuximab resistance in head and neck cancer.PLoS One. 2020 Feb 18;15(2):e0229077. doi: 10.1371/journal.pone.0229077. eCollection 2020. PLoS One. 2020. PMID: 32069320 Free PMC article.
-
ASCT2 (SLC1A5) is an EGFR-associated protein that can be co-targeted by cetuximab to sensitize cancer cells to ROS-induced apoptosis.Cancer Lett. 2016 Oct 10;381(1):23-30. doi: 10.1016/j.canlet.2016.07.020. Epub 2016 Jul 19. Cancer Lett. 2016. PMID: 27450723 Free PMC article.
-
Decreased SMAD4 expression is associated with induction of epithelial-to-mesenchymal transition and cetuximab resistance in head and neck squamous cell carcinoma.Cancer Biol Ther. 2015;16(8):1252-8. doi: 10.1080/15384047.2015.1056418. Epub 2015 Jun 5. Cancer Biol Ther. 2015. PMID: 26046389 Free PMC article.
-
The role of cetuximab in the treatment of squamous cell cancer of the head and neck.Expert Opin Biol Ther. 2005 Aug;5(8):1085-93. doi: 10.1517/14712598.5.8.1085. Expert Opin Biol Ther. 2005. PMID: 16050785 Review.
-
Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.Drugs Today (Barc). 2005 Feb;41(2):107-27. doi: 10.1358/dot.2005.41.2.882662. Drugs Today (Barc). 2005. PMID: 15821783 Review.
Cited by
-
CYLD in health and disease.Dis Model Mech. 2023 Jun 1;16(6):dmm050093. doi: 10.1242/dmm.050093. Epub 2023 Jun 30. Dis Model Mech. 2023. PMID: 37387450 Free PMC article. Review.
-
Potential use of EGFR-targeted molecular therapies for tumor suppressor CYLD-negative and poor prognosis oral squamous cell carcinoma with chemoresistance.Cancer Cell Int. 2022 Nov 15;22(1):358. doi: 10.1186/s12935-022-02781-x. Cancer Cell Int. 2022. PMID: 36376983 Free PMC article.
-
Effective combination treatments for breast cancer inhibition by FOXM1 inhibitors with other targeted cancer drugs.Breast Cancer Res Treat. 2023 Apr;198(3):607-621. doi: 10.1007/s10549-023-06878-3. Epub 2023 Feb 27. Breast Cancer Res Treat. 2023. PMID: 36847915
-
Chondroitin polymerizing factor predicts a poor prognosis and promotes breast cancer progression via the upstream TGF-β1/SMAD3 and JNK axis activation.J Cell Commun Signal. 2023 Mar;17(1):89-102. doi: 10.1007/s12079-022-00684-0. Epub 2022 Aug 30. J Cell Commun Signal. 2023. PMID: 36042157 Free PMC article.
-
The role of FoxM1 in immune cells.Clin Exp Med. 2023 Oct;23(6):1973-1979. doi: 10.1007/s10238-023-01037-w. Epub 2023 Mar 13. Clin Exp Med. 2023. PMID: 36913035 Review.
References
-
- van der Heijden M., Essers P., de Jong M.C., de Roest R.H., Sanduleanu S., Verhagen C.V., Vens C. Biological determinants of chemo-radiotherapy response in HPV-negative head and neck cancer: A multicentric external validation. Front. Oncol. 2020;9:1470. doi: 10.3389/fonc.2019.01470. - DOI - PMC - PubMed
-
- Machiels J.P., Leemans C.R., Golusinski W., Grau C., Licitra L., Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1462–1475. doi: 10.1016/j.annonc.2020.07.011. - DOI - PubMed
-
- Lerch S., Berthold S., Ziemann F., Dreffke K., Subtil F.S., Senger Y., Jensen A., Engenhart-Cabillic R., Dikomey E., Wittig A., et al. HPV-positive HNSCC cell lines show strongly enhanced radiosensitivity after photon but not after carbon ion irradiation. Radiother. Oncol. 2020;151:134–140. doi: 10.1016/j.radonc.2020.07.032. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

