GATA2 deficiency elevates interferon regulatory factor-8 to subvert a progenitor cell differentiation program
- PMID: 35008108
- PMCID: PMC8905696
- DOI: 10.1182/bloodadvances.2021006182
GATA2 deficiency elevates interferon regulatory factor-8 to subvert a progenitor cell differentiation program
Abstract
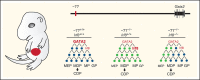
Cell type-specific transcription factors control stem and progenitor cell transitions by establishing networks containing hundreds of genes and proteins. Network complexity renders it challenging to discover essential versus modulatory or redundant components. This scenario is exemplified by GATA2 regulation of hematopoiesis during embryogenesis. Loss of a far upstream Gata2 enhancer (-77) disrupts the GATA2-dependent transcriptome governing hematopoietic progenitor cell differentiation. The aberrant transcriptome includes the transcription factor interferon regulatory factor 8 (IRF8) and a host of innate immune regulators. Mutant progenitors lose the capacity to balance production of diverse hematopoietic progeny. To elucidate mechanisms, we asked if IRF8 is essential, contributory, or not required. Reducing Irf8, in the context of the -77 mutant allele, reversed granulocytic deficiencies and the excessive accumulation of dendritic cell committed progenitors. Despite many dysregulated components that control vital transcriptional, signaling, and immune processes, the aberrant elevation of a single transcription factor deconstructed the differentiation program.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures




Similar articles
-
Pathogenic human variant that dislocates GATA2 zinc fingers disrupts hematopoietic gene expression and signaling networks.J Clin Invest. 2023 Apr 3;133(7):e162685. doi: 10.1172/JCI162685. J Clin Invest. 2023. PMID: 36809258 Free PMC article.
-
Interferon regulatory factor-8-dependent innate immune alarm senses GATA2 deficiency to alter hematopoietic differentiation and function.Curr Opin Hematol. 2023 Jul 1;30(4):117-123. doi: 10.1097/MOH.0000000000000763. Epub 2023 Apr 27. Curr Opin Hematol. 2023. PMID: 37254854 Free PMC article. Review.
-
Sequencing of RNA in single cells reveals a distinct transcriptome signature of hematopoiesis in GATA2 deficiency.Blood Adv. 2020 Jun 23;4(12):2656-2670. doi: 10.1182/bloodadvances.2019001352. Blood Adv. 2020. PMID: 32556286 Free PMC article.
-
GATA2 deficiency and human hematopoietic development modeled using induced pluripotent stem cells.Blood Adv. 2018 Dec 11;2(23):3553-3565. doi: 10.1182/bloodadvances.2018017137. Blood Adv. 2018. PMID: 30538114 Free PMC article.
-
The role of the GATA2 transcription factor in normal and malignant hematopoiesis.Crit Rev Oncol Hematol. 2012 Apr;82(1):1-17. doi: 10.1016/j.critrevonc.2011.04.007. Epub 2011 May 24. Crit Rev Oncol Hematol. 2012. PMID: 21605981 Review.
Cited by
-
Linking GATA2 to myeloid dysplasia and complex cytogenetics in adult myelodysplastic neoplasm and acute myeloid leukemia.Blood Adv. 2024 Jan 9;8(1):80-92. doi: 10.1182/bloodadvances.2023011554. Blood Adv. 2024. PMID: 38029365 Free PMC article.
-
Restricting genomic actions of innate immune mediators on fetal hematopoietic progenitor cells.iScience. 2023 Feb 28;26(4):106297. doi: 10.1016/j.isci.2023.106297. eCollection 2023 Apr 21. iScience. 2023. PMID: 36950124 Free PMC article.
-
TET2 lesions enhance the aggressiveness of CEBPA-mutant acute myeloid leukemia by rebalancing GATA2 expression.Nat Commun. 2023 Oct 4;14(1):6185. doi: 10.1038/s41467-023-41927-x. Nat Commun. 2023. PMID: 37794021 Free PMC article.
-
Pathogenic GATA2 genetic variants utilize an obligate enhancer mechanism to distort a multilineage differentiation program.Proc Natl Acad Sci U S A. 2024 Mar 5;121(10):e2317147121. doi: 10.1073/pnas.2317147121. Epub 2024 Feb 29. Proc Natl Acad Sci U S A. 2024. PMID: 38422019 Free PMC article.
-
Pathogenic human variant that dislocates GATA2 zinc fingers disrupts hematopoietic gene expression and signaling networks.J Clin Invest. 2023 Apr 3;133(7):e162685. doi: 10.1172/JCI162685. J Clin Invest. 2023. PMID: 36809258 Free PMC article.
References
-
- Tsai SF, Martin DI, Zon LI, D’Andrea AD, Wong GG, Orkin SH. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339(6224):446-451. - PubMed
-
- Tsai FY, Keller G, Kuo FC, et al. . An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371(6494):221-226. - PubMed
-
- Evans T, Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989;58(5):877-885. - PubMed
-
- Tsai F-Y, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89(10):3636-3643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

