Transcranial Ultrasound Stimulation of the Anterior Cingulate Cortex Reduces Neuropathic Pain in Mice
- PMID: 35003307
- PMCID: PMC8741380
- DOI: 10.1155/2021/6510383
Transcranial Ultrasound Stimulation of the Anterior Cingulate Cortex Reduces Neuropathic Pain in Mice
Abstract
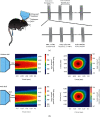
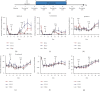
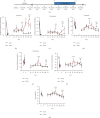
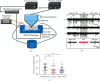
Focused ultrasound (FUS) is a potential tool for treating chronic pain by modulating the central nervous system. Herein, we aimed to determine whether transcranial FUS stimulation of the anterior cingulate cortex (ACC) effectively improved chronic pain in the chronic compress injury mice model at different stages of neuropathic pain. The mechanical threshold of pain was recorded in the nociceptive tests. We found FUS stimulation elevated the mechanical threshold of pain in both short-term (p < 0.01) and long-term (p < 0.05) experiments. Furthermore, we determined protein expression differences in ACC between the control group, the intervention group, and the Sham group to analyze the underlying mechanism of FUS stimulation in improving neuropathic pain. Additionally, the results showed FUS stimulation led to alterations in differential proteins in long-term experiments, including cellular processes, cellular signaling, and information storage and processing. Our findings indicate FUS may effectively alleviate mechanical neuropathic pain via the ACC's stimulation, especially in the chronic state.
Copyright © 2021 Xiangjun Feng et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this study.
Figures


Similar articles
-
External focused ultrasound treatment for neuropathic pain induced by common peroneal nerve injury.Neurosci Lett. 2018 Sep 25;684:145-151. doi: 10.1016/j.neulet.2018.07.037. Epub 2018 Jul 26. Neurosci Lett. 2018. PMID: 30056105
-
Optical inactivation of the anterior cingulate cortex modulate descending pain pathway in a rat model of trigeminal neuropathic pain created via chronic constriction injury of the infraorbital nerve.J Pain Res. 2017 Oct 3;10:2355-2364. doi: 10.2147/JPR.S138626. eCollection 2017. J Pain Res. 2017. PMID: 29042811 Free PMC article.
-
Low-Energy Transcranial Navigation-Guided Focused Ultrasound for Neuropathic Pain: An Exploratory Study.Brain Sci. 2023 Oct 8;13(10):1433. doi: 10.3390/brainsci13101433. Brain Sci. 2023. PMID: 37891801 Free PMC article.
-
Deep brain stimulation of the dorsal anterior cingulate cortex for the treatment of chronic neuropathic pain.Neurosurg Focus. 2015 Jun;38(6):E11. doi: 10.3171/2015.3.FOCUS1543. Neurosurg Focus. 2015. PMID: 26030699 Review.
-
Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway.Mol Pain. 2009 Sep 4;5:51. doi: 10.1186/1744-8069-5-51. Mol Pain. 2009. PMID: 19732417 Free PMC article. Review.
Cited by
-
Long non-coding RNA rhabdomyosarcoma 2-associated transcript contributes to neuropathic pain by recruiting HuR to stabilize DNA methyltransferase 3 alpha mRNA expression in dorsal root ganglion neuron.Front Mol Neurosci. 2023 Feb 22;15:1027063. doi: 10.3389/fnmol.2022.1027063. eCollection 2022. Front Mol Neurosci. 2023. PMID: 36911851 Free PMC article.
-
Multimodal non-invasive non-pharmacological therapies for chronic pain: mechanisms and progress.BMC Med. 2023 Sep 29;21(1):372. doi: 10.1186/s12916-023-03076-2. BMC Med. 2023. PMID: 37775758 Free PMC article. Review.
-
RNA interference-mediated silencing of DNA methyltransferase 1 attenuates neuropathic pain by accelerating microglia M2 polarization.BMC Neurol. 2022 Oct 1;22(1):376. doi: 10.1186/s12883-022-02860-6. BMC Neurol. 2022. PMID: 36183073 Free PMC article.
-
The Emergence of Functional Ultrasound for Noninvasive Brain-Computer Interface.Research (Wash D C). 2023 Aug 15;6:0200. doi: 10.34133/research.0200. eCollection 2023. Research (Wash D C). 2023. PMID: 37588619 Free PMC article. Review.
-
Deep Brain Stimulation, Stereotactic Radiosurgery and High-Intensity Focused Ultrasound Targeting the Limbic Pain Matrix: A Comprehensive Review.Pain Ther. 2022 Jun;11(2):459-476. doi: 10.1007/s40122-022-00381-1. Epub 2022 Apr 26. Pain Ther. 2022. PMID: 35471626 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

