Apoptosis, Autophagy, NETosis, Necroptosis, and Pyroptosis Mediated Programmed Cell Death as Targets for Innovative Therapy in Rheumatoid Arthritis
- PMID: 35003139
- PMCID: PMC8739882
- DOI: 10.3389/fimmu.2021.809806
Apoptosis, Autophagy, NETosis, Necroptosis, and Pyroptosis Mediated Programmed Cell Death as Targets for Innovative Therapy in Rheumatoid Arthritis
Abstract
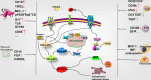
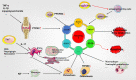
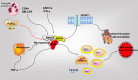
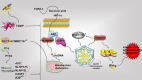
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease that can lead to clinical manifestations of systemic diseases. Its leading features include chronic synovial inflammation and degeneration of the bones and joints. In the past decades, multiple susceptibilities for rheumatoid arthritis have been identified along with the development of a remarkable variety of drugs for its treatment; which include analgesics, glucocorticoids, nonsteroidal anti-inflammatory medications (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs), and biologic response modifiers (bDMARDs). Despite the existence of many clinical treatment options, the prognosis of some patients remains poor due to complex mechanism of the disease. Programmed cell death (PCD) has been extensively studied and ascertained to be one of the essential pathological mechanisms of RA. Its dysregulation in various associated cell types contributes to the development of RA. In this review, we summarize the role of apoptosis, cell death-associated neutrophil extracellular trap formation, necroptosis, pyroptosis, and autophagy in the pathophysiology of RA to provide a theoretical reference and insightful direction to the discovery and development of novel therapeutic targets for RA.
Keywords: NETosis; apoptosis; autophagy; necroptosis; programmed cell death; pyroptosis; rheumatoid arthritis.
Copyright © 2021 Zhao, Jiang, Guo, Schrodi and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
[The research development of programmed cell death in rheumatoid arthritis].Sheng Li Xue Bao. 2024 Oct 25;76(5):827-840. Sheng Li Xue Bao. 2024. PMID: 39468819 Review. Chinese.
-
Can pyroptosis be a new target in rheumatoid arthritis treatment?Front Immunol. 2023 Jun 22;14:1155606. doi: 10.3389/fimmu.2023.1155606. eCollection 2023. Front Immunol. 2023. PMID: 37426634 Free PMC article. Review.
-
Acid-Sensing Ion Channel-1a in Articular Chondrocytes and Synovial Fibroblasts: A Novel Therapeutic Target for Rheumatoid Arthritis.Front Immunol. 2021 Jan 28;11:580936. doi: 10.3389/fimmu.2020.580936. eCollection 2020. Front Immunol. 2021. PMID: 33584647 Free PMC article. Review.
-
Pyroptosis in periodontitis: From the intricate interaction with apoptosis, NETosis, and necroptosis to the therapeutic prospects.Front Cell Infect Microbiol. 2022 Aug 16;12:953277. doi: 10.3389/fcimb.2022.953277. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36093182 Free PMC article. Review.
-
Emodin ameliorates rheumatoid arthritis by promoting neutrophil apoptosis and inhibiting neutrophil extracellular trap formation.Mol Immunol. 2019 Aug;112:188-197. doi: 10.1016/j.molimm.2019.05.010. Epub 2019 Jun 5. Mol Immunol. 2019. PMID: 31176198
Cited by
-
Cuproptosis in ccRCC: key player in therapeutic and prognostic targets.Front Oncol. 2023 Oct 27;13:1271864. doi: 10.3389/fonc.2023.1271864. eCollection 2023. Front Oncol. 2023. PMID: 37965478 Free PMC article.
-
The role of anti-citrullinated protein antibody in pathogenesis of RA.Clin Exp Med. 2024 Jul 8;24(1):153. doi: 10.1007/s10238-024-01359-3. Clin Exp Med. 2024. PMID: 38972923 Free PMC article. Review.
-
DNA Methylation of T Lymphocytes as a Therapeutic Target: Implications for Rheumatoid Arthritis Etiology.Front Immunol. 2022 Mar 3;13:863703. doi: 10.3389/fimmu.2022.863703. eCollection 2022. Front Immunol. 2022. PMID: 35309322 Free PMC article. Review.
-
Development of an In Vitro Model for Inflammation Mediated Renal Toxicity Using 3D Renal Tubules and Co-Cultured Human Immune Cells.Tissue Eng Regen Med. 2023 Dec;20(7):1173-1190. doi: 10.1007/s13770-023-00602-4. Epub 2023 Oct 16. Tissue Eng Regen Med. 2023. PMID: 37843784 Free PMC article.
-
Downregulation of BIRC2 hinders the progression of rheumatoid arthritis through regulating TRADD.Immun Inflamm Dis. 2023 Oct;11(10):e978. doi: 10.1002/iid3.978. Immun Inflamm Dis. 2023. PMID: 37904685 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

