Response and Duration of Serum Anti-SARS-CoV-2 Antibodies After Inactivated Vaccination Within 160 Days
- PMID: 35003104
- PMCID: PMC8733590
- DOI: 10.3389/fimmu.2021.786554
Response and Duration of Serum Anti-SARS-CoV-2 Antibodies After Inactivated Vaccination Within 160 Days
Abstract
Background: A vaccine against coronavirus disease 2019 (COVID-19) with highly effective protection is urgently needed. The anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody response and duration after vaccination are crucial predictive indicators.
Objectives: To evaluate the response and duration for 5 subsets of anti-SARS-CoV-2 antibodies after vaccination and their predictive value for protection.
Methods: We determined the response and duration for 5 subsets of anti-SARS-CoV-2 antibodies (neutralizing antibody, anti-RBD total antibody, anti-Spike IgG, anti-Spike IgM, and anti-Spike IgA) in 61 volunteers within 160 days after the CoronaVac vaccine. A logistic regression model was used to determine the predictors of the persistence of neutralizing antibody persistence.
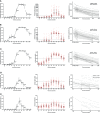
Results: The seropositivity rates of neutralizing antibody, anti-RBD total antibody, anti-Spike IgG, anti-Spike IgM, and anti-Spike IgA were only 4.92%, 27.87%, 21.31%, 3.28% and 0.00%, respectively, at the end of the first dose (28 days). After the second dose, the seropositivity rates reached peaks of 95.08%, 100.00%, 100.00%, 59.02% and 31.15% in two weeks (42 days). Their decay was obvious and the seropositivity rate remained at 19.67%, 54.10%, 50.82%, 3.28% and 0.00% on day 160, respectively. The level of neutralizing antibody reached a peak of 149.40 (101.00-244.60) IU/mL two weeks after the second dose (42 days) and dropped to 14.23 (7.62-30.73) IU/mL at 160 days, with a half-life of 35.61(95% CI, 32.68 to 39.12) days. Younger participants (≤31 years) had 6.179 times more persistent neutralizing antibodies than older participants (>31 years) (P<0.05). Participants with anti-Spike IgA seropositivity had 4.314 times greater persistence of neutralizing antibodies than participants without anti-Spike IgA seroconversion (P<0.05).
Conclusions: Antibody response for the CoronaVac vaccine was intense and comprehensive with 95.08% neutralizing seropositivity rate, while decay was also obvious after 160 days. Therefore, booster doses should be considered in the vaccine strategies.
Keywords: COVID-19; CoronaVac; SARS-CoV-2; anti-SARS-CoV-2 antibody; neutralizing antibody.
Copyright © 2021 Xu, Xue, Xiao, Jia, Wu, Liu, Li, Liang and Yang.
Conflict of interest statement
Authors Z-JJ, MJ-W, and Y-YL are employed by Xiamen Boson Biotech Co., Ltd., and author W-LL is employed by Autobio Diagnostic Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
 Receive vaccine .
Receive vaccine .Similar articles
-
A Third Dose of an Inactivated Vaccine Dramatically Increased the Levels and Decay Times of Anti-SARS-CoV-2 Antibodies, but Disappointingly Declined Again: A Prospective, Longitudinal, Cohort Study at 18 Serial Time Points Over 368 Days.Front Immunol. 2022 Apr 29;13:876037. doi: 10.3389/fimmu.2022.876037. eCollection 2022. Front Immunol. 2022. PMID: 35572536 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen.J Med Virol. 2022 Jan;94(1):39-41. doi: 10.1002/jmv.27350. Epub 2021 Sep 21. J Med Virol. 2022. PMID: 34536028 No abstract available.
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
-
Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence.Viruses. 2022 Jan 19;14(2):187. doi: 10.3390/v14020187. Viruses. 2022. PMID: 35215783 Free PMC article. Review.
Cited by
-
Efficacy of Six Different SARS-CoV-2 Vaccines during a Six-Month Follow-Up and Five COVID-19 Waves in Brazil and Mexico.Vaccines (Basel). 2023 Apr 14;11(4):842. doi: 10.3390/vaccines11040842. Vaccines (Basel). 2023. PMID: 37112754 Free PMC article.
-
Evaluation of the efficacy of four anti-SARS-CoV-2 antibodies after vaccination using kits from two manufacturers: A prospective, longitudinal, cohort study at 11 serial time points within 160 days.Int Immunopharmacol. 2022 Nov;112:109285. doi: 10.1016/j.intimp.2022.109285. Epub 2022 Sep 28. Int Immunopharmacol. 2022. PMID: 36182874 Free PMC article.
-
A Third Dose of an Inactivated Vaccine Dramatically Increased the Levels and Decay Times of Anti-SARS-CoV-2 Antibodies, but Disappointingly Declined Again: A Prospective, Longitudinal, Cohort Study at 18 Serial Time Points Over 368 Days.Front Immunol. 2022 Apr 29;13:876037. doi: 10.3389/fimmu.2022.876037. eCollection 2022. Front Immunol. 2022. PMID: 35572536 Free PMC article.
-
Is the fourth COVID-19 vaccine dose urgently needed? Revelation from a prospective cohort study.J Infect. 2022 Sep;85(3):e66-e68. doi: 10.1016/j.jinf.2022.06.003. Epub 2022 Jun 9. J Infect. 2022. PMID: 35691516 Free PMC article. No abstract available.
-
Low Peripheral B-Cell Counts in Patients With Systemic Rheumatic Diseases Due to Treatment With Belimumab and/or Rituximab Are Associated With Low Antibody Responses to Primary COVID-19 Vaccination.HSS J. 2023 May;19(2):180-186. doi: 10.1177/15563316221142846. Epub 2022 Dec 16. HSS J. 2023. PMID: 37051614 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

