Mechanobiology of Microvascular Function and Structure in Health and Disease: Focus on the Coronary Circulation
- PMID: 35002759
- PMCID: PMC8733629
- DOI: 10.3389/fphys.2021.771960
Mechanobiology of Microvascular Function and Structure in Health and Disease: Focus on the Coronary Circulation
Abstract
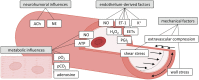
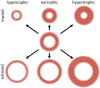
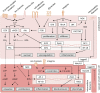
The coronary microvasculature plays a key role in regulating the tight coupling between myocardial perfusion and myocardial oxygen demand across a wide range of cardiac activity. Short-term regulation of coronary blood flow in response to metabolic stimuli is achieved via adjustment of vascular diameter in different segments of the microvasculature in conjunction with mechanical forces eliciting myogenic and flow-mediated vasodilation. In contrast, chronic adjustments in flow regulation also involve microvascular structural modifications, termed remodeling. Vascular remodeling encompasses changes in microvascular diameter and/or density being largely modulated by mechanical forces acting on the endothelium and vascular smooth muscle cells. Whereas in recent years, substantial knowledge has been gathered regarding the molecular mechanisms controlling microvascular tone and how these are altered in various diseases, the structural adaptations in response to pathologic situations are less well understood. In this article, we review the factors involved in coronary microvascular functional and structural alterations in obstructive and non-obstructive coronary artery disease and the molecular mechanisms involved therein with a focus on mechanobiology. Cardiovascular risk factors including metabolic dysregulation, hypercholesterolemia, hypertension and aging have been shown to induce microvascular (endothelial) dysfunction and vascular remodeling. Additionally, alterations in biomechanical forces produced by a coronary artery stenosis are associated with microvascular functional and structural alterations. Future studies should be directed at further unraveling the mechanisms underlying the coronary microvascular functional and structural alterations in disease; a deeper understanding of these mechanisms is critical for the identification of potential new targets for the treatment of ischemic heart disease.
Keywords: coronary blood flow; endothelial dysfunction; ischemic heart disease; microvascular density; microvascular disease; microvascular dysfunction; microvascular remodeling.
Copyright © 2021 Brandt, Cheng, Merkus, Duncker and Sorop.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Regulation of coronary blood flow during exercise.Physiol Rev. 2008 Jul;88(3):1009-86. doi: 10.1152/physrev.00045.2006. Physiol Rev. 2008. PMID: 18626066 Review.
-
Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis.Circ Res. 2008 Apr 11;102(7):795-803. doi: 10.1161/CIRCRESAHA.108.172528. Epub 2008 Feb 21. Circ Res. 2008. PMID: 18292598
-
Reappraisal of Ischemic Heart Disease.Circulation. 2018 Oct 2;138(14):1463-1480. doi: 10.1161/CIRCULATIONAHA.118.031373. Circulation. 2018. PMID: 30354347 Review.
-
Critical role of the coronary microvasculature in heart disease: From pathologic driving force to "innocent" bystander.Am Heart J Plus. 2022 Oct 1;22:100215. doi: 10.1016/j.ahjo.2022.100215. eCollection 2022 Oct. Am Heart J Plus. 2022. PMID: 38558907 Free PMC article.
-
[Coronary microvascular disease: from experimental models to clinical practice].Recenti Prog Med. 2012 Jul-Aug;103(7-8):288-96. doi: 10.1701/1127.12433. Recenti Prog Med. 2012. PMID: 22825385 Review. Italian.
Cited by
-
Canagliflozin improves coronary microvascular vasodilation and increases absolute blood flow to the myocardium independent of angiogenesis.J Thorac Cardiovasc Surg. 2023 Dec;166(6):e535-e550. doi: 10.1016/j.jtcvs.2023.08.017. Epub 2023 Aug 20. J Thorac Cardiovasc Surg. 2023. PMID: 37604273 Free PMC article.
-
Radiation induces acute and subacute vascular regression in a three-dimensional microvasculature model.Front Oncol. 2023 Oct 16;13:1252014. doi: 10.3389/fonc.2023.1252014. eCollection 2023. Front Oncol. 2023. PMID: 37909014 Free PMC article.
-
Myocardial perfusion improvement and mechanism after percutaneous intramyocardial septal radiofrequency ablation in obstructive hypertrophic cardiomyopathy: a study of myocardial contrast echocardiography.Int J Cardiovasc Imaging. 2024 Jul;40(7):1483-1492. doi: 10.1007/s10554-024-03126-7. Epub 2024 May 6. Int J Cardiovasc Imaging. 2024. PMID: 38709352
-
Collagen IV deficiency causes hypertrophic remodeling and endothelium-dependent hyperpolarization in small vessel disease with intracerebral hemorrhage.EBioMedicine. 2024 Sep;107:105315. doi: 10.1016/j.ebiom.2024.105315. Epub 2024 Aug 30. EBioMedicine. 2024. PMID: 39216230 Free PMC article.
-
The development of peripheral microvasculopathy with chronic metabolic disease in obese Zucker rats: a retrograde emergence?Am J Physiol Heart Circ Physiol. 2022 Sep 1;323(3):H475-H489. doi: 10.1152/ajpheart.00264.2022. Epub 2022 Jul 29. Am J Physiol Heart Circ Physiol. 2022. PMID: 35904886 Free PMC article.
References
-
- Adler A., Messina E., Sherman B., Wang Z., Huang H., Linke A., et al. . (2003). NAD(P)H oxidase-generated superoxide anion accounts for reduced control of myocardial O2 consumption by NO in old Fischer 344 rats. Am. J. Physiol. Heart Circ. Physiol. 285, H1015–H1022. doi: 10.1152/ajpheart.01047.2002, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

