Stem Cell Mimicking Nanoencapsulation for Targeting Arthritis
- PMID: 35002240
- PMCID: PMC8725870
- DOI: 10.2147/IJN.S334298
Stem Cell Mimicking Nanoencapsulation for Targeting Arthritis
Abstract
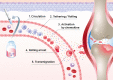
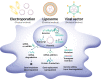
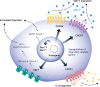
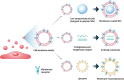
Mesenchymal stem cells (MSCs) are considered a promising regenerative therapy due to their ability to migrate toward damaged tissues. The homing ability of MSCs is unique compared with that of non-migrating cells and MSCs are considered promising therapeutic vectors for targeting major cells in many pathophysiological sites. MSCs have many advantages in the treatment of malignant diseases, particularly rheumatoid arthritis (RA). RA is a representative autoimmune disease that primarily affects joints, and secreted chemokines in the joints are well recognized by MSCs following their migration to the joints. Furthermore, MSCs can regulate the inflammatory process and repair damaged cells in the joints. However, the functionality and migration ability of MSCs injected in vivo still show insufficient. The targeting ability and migration efficiency of MSCs can be enhanced by genetic engineering or modification, eg, overexpressing chemokine receptors or migration-related genes, thus maximizing their therapeutic effect. However, there are concerns about genetic changes due to the increased probability of oncogenesis resulting from genome integration of the viral vector, and thus, clinical application is limited. Furthermore, it is suspected that administering MSCs can promote tumor growth and metastasis in xenograft and orthotopic models. For this reason, MSC mimicking nanoencapsulations are an alternative strategy that does not involve using MSCs or bioengineered MSCs. MSC mimicking nanoencapsulations consist of MSC membrane-coated nanoparticles, MSC-derived exosomes and artificial ectosomes, and MSC membrane-fused liposomes with natural or genetically engineered MSC membranes. MSC mimicking nanoencapsulations not only retain the targeting ability of MSCs but also have many advantages in terms of targeted drug delivery. Specifically, MSC mimicking nanoencapsulations are capable of encapsulating drugs with various components, including chemotherapeutic agents, nucleic acids, and proteins. Furthermore, there are fewer concerns over safety issues on MSC mimicking nanoencapsulations associated with mutagenesis even when using genetically engineered MSCs, because MSC mimicking nanoencapsulations use only the membrane fraction of MSCs. Genetic engineering is a promising route in clinical settings, where nano-encapsulated technology strategies are combined. In this review, the mechanism underlying MSC homing and the advantages of MSC mimicking nanoencapsulations are discussed. In addition, genetic engineering of MSCs and MSC mimicking nanoencapsulation is described as a promising strategy for the treatment of immune-related diseases.
Keywords: autoimmune disease targeting strategy; ectosomes; exosomes; liposomes; stem cell migration; stem cell mimicking nanoencapsulations.
© 2021 Shin et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






Similar articles
-
miRNA-146a Improves Immunomodulatory Effects of MSC-derived Exosomes in Rheumatoid Arthritis.Curr Gene Ther. 2020;20(4):297-312. doi: 10.2174/1566523220666200916120708. Curr Gene Ther. 2020. PMID: 32938348
-
Inflammation-Targeting Mesenchymal Stem Cells Combined with Photothermal Treatment Attenuate Severe Joint Inflammation.Adv Mater. 2024 Mar;36(11):e2304333. doi: 10.1002/adma.202304333. Epub 2023 Dec 22. Adv Mater. 2024. PMID: 38096399
-
Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis.Front Immunol. 2020 Aug 20;11:1912. doi: 10.3389/fimmu.2020.01912. eCollection 2020. Front Immunol. 2020. PMID: 32973792 Free PMC article. Review.
-
Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin.Int J Med Sci. 2018 Jun 22;15(10):1051-1061. doi: 10.7150/ijms.25760. eCollection 2018. Int J Med Sci. 2018. PMID: 30013447 Free PMC article.
-
Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis.Rheumatology (Oxford). 2015 Feb;54(2):210-8. doi: 10.1093/rheumatology/keu377. Epub 2014 Oct 6. Rheumatology (Oxford). 2015. PMID: 25288785 Review.
Cited by
-
Cell therapy in Sjögren's syndrome: opportunities and challenges.Expert Rev Mol Med. 2024 Oct 23;26:e28. doi: 10.1017/erm.2024.21. Expert Rev Mol Med. 2024. PMID: 39438246 Free PMC article. Review.
-
Exosomes derived from fibrinogen-like protein 1-overexpressing bone marrow-derived mesenchymal stem cells ameliorates rheumatoid arthritis.Bioengineered. 2022 Jun;13(6):14545-14561. doi: 10.1080/21655979.2022.2090379. Bioengineered. 2022. PMID: 36694465 Free PMC article.
-
Liposomes for Tumor Targeted Therapy: A Review.Int J Mol Sci. 2023 Jan 31;24(3):2643. doi: 10.3390/ijms24032643. Int J Mol Sci. 2023. PMID: 36768966 Free PMC article. Review.
-
Therapeutic Applications of Mesenchymal Stem Cell Loaded with Gold Nanoparticles for Regenerative Medicine.Pharmaceutics. 2023 Apr 30;15(5):1385. doi: 10.3390/pharmaceutics15051385. Pharmaceutics. 2023. PMID: 37242627 Free PMC article.
-
Exosomes in the Treatment of Pancreatic Cancer: A Moonshot to PDAC Treatment?Int J Mol Sci. 2022 Mar 25;23(7):3620. doi: 10.3390/ijms23073620. Int J Mol Sci. 2022. PMID: 35408980 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

