Recent advances in systemic therapy for hepatocellular carcinoma
- PMID: 35000616
- PMCID: PMC8744248
- DOI: 10.1186/s40364-021-00350-4
Recent advances in systemic therapy for hepatocellular carcinoma
Abstract
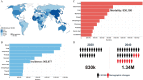
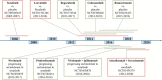
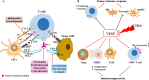
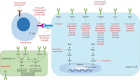
Hepatocellular carcinoma (HCC) is one of the most common and lethal malignant tumors in the world. Therapeutic options for advanced HCC are limited. Systemic treatment, especially with conventional cytotoxic drugs, is usually ineffective. For more than a decade, sorafenib has been the only systemic drug that has been proven to be clinically effective for treating advanced HCC. However, over the past three years, the rapid progress of molecular targeted therapies has dramatically changed the treatment landscape for advanced HCC. Immune checkpoint therapies are now being incorporated into HCC therapies, and their combination with molecular targeted therapy is emerging as a tool to enhance the immune response. In this review, we summarize the development and progress of molecular targeted agents and immunotherapies in HCC.
Keywords: Combination; Hepatocellular carcinoma; Immunotherapies; Molecular targeted therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Recent Update on Immunotherapy and Its Combination With Interventional Therapies for Hepatocellular Carcinoma.Clin Med Insights Oncol. 2022 Nov 11;16:11795549221134832. doi: 10.1177/11795549221134832. eCollection 2022. Clin Med Insights Oncol. 2022. PMID: 36387611 Free PMC article. Review.
-
Recent Progress in Systemic Therapy for Hepatocellular Cancer (HCC).Curr Treat Options Gastroenterol. 2021 Jun;19(2):351-368. doi: 10.1007/s11938-021-00346-x. Epub 2021 Mar 31. Curr Treat Options Gastroenterol. 2021. PMID: 35530750 Free PMC article.
-
Systemic therapy for advanced hepatocellular carcinoma: targeted therapies.Chin Clin Oncol. 2021 Feb;10(1):10. doi: 10.21037/cco-20-117. Epub 2020 May 14. Chin Clin Oncol. 2021. PMID: 32434345
-
Evolving therapeutic landscape of advanced hepatocellular carcinoma.Nat Rev Gastroenterol Hepatol. 2023 Apr;20(4):203-222. doi: 10.1038/s41575-022-00704-9. Epub 2022 Nov 11. Nat Rev Gastroenterol Hepatol. 2023. PMID: 36369487 Review.
-
Current statuses of molecular targeted and immune checkpoint therapies in hepatocellular carcinoma.Am J Cancer Res. 2020 May 1;10(5):1522-1533. eCollection 2020. Am J Cancer Res. 2020. PMID: 32509395 Free PMC article.
Cited by
-
EGR1 suppresses HCC growth and aerobic glycolysis by transcriptionally downregulating PFKL.J Exp Clin Cancer Res. 2024 Jan 29;43(1):35. doi: 10.1186/s13046-024-02957-5. J Exp Clin Cancer Res. 2024. PMID: 38287371 Free PMC article.
-
New perspectives on chemokines in hepatocellular carcinoma therapy: a critical pathway for natural products regulation of the tumor microenvironment.Front Immunol. 2024 Aug 14;15:1456405. doi: 10.3389/fimmu.2024.1456405. eCollection 2024. Front Immunol. 2024. PMID: 39206194 Free PMC article. Review.
-
Pomegranate Peel Extract Sensitizes Hepatocellular Carcinoma Cells to Ionizing Radiation, Induces Apoptosis and Inhibits MAPK, JAK/STAT3, β-Catenin/NOTCH, and SOCS3 Signaling.Integr Cancer Ther. 2023 Jan-Dec;22:15347354221151021. doi: 10.1177/15347354221151021. Integr Cancer Ther. 2023. PMID: 36710483 Free PMC article.
-
Design and synthesis of ludartin derivatives as potential anticancer agents against hepatocellular carcinoma.Med Chem Res. 2022;31(7):1224-1239. doi: 10.1007/s00044-022-02890-2. Epub 2022 May 24. Med Chem Res. 2022. PMID: 35634434 Free PMC article.
-
A Comprehensive Narrative Review on the History, Current Landscape, and Future Directions of Hepatocellular Carcinoma (HCC) Systemic Therapy.Cancers (Basel). 2023 Apr 27;15(9):2506. doi: 10.3390/cancers15092506. Cancers (Basel). 2023. PMID: 37173972 Free PMC article. Review.
References
-
- Cancer Today [https://gco.iarc.fr/today/home]. Accessed 20 Feb 2021.
-
- Cancer Tomorrow [https://gco.iarc.fr/tomorrow]. Accessed 20 Feb 2021.
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Sim HW, Knox J. Hepatocellular carcinoma in the era of immunotherapy. Curr Probl Cancer. 2018;42(1):40–48. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

