IL-25 Treatment Improves Metabolic Syndrome in High-Fat Diet and Genetic Models of Obesity
- PMID: 34992396
- PMCID: PMC8710075
- DOI: 10.2147/DMSO.S335761
IL-25 Treatment Improves Metabolic Syndrome in High-Fat Diet and Genetic Models of Obesity
Abstract
Introduction: Endemic obesity is considered the driving force for the dramatic increase in incidence of type 2 diabetes (T2D). There is mounting evidence that chronic, low-grade inflammation driven by Th1/Th17 cells and M1 macrophages, is a critical link between obesity and insulin resistance. IL-25 promotes development of a Th2 immune response and M2 macrophages that counteract the inflammation associated with obesity and T2D.
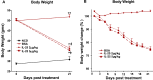
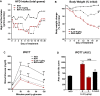
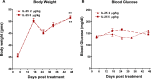
Methods: Mice were fed a high-fat diet (HFD) for 16 weeks and then treated with IL-25 or BSA as a control for 21 days. Body weight, blood glucose levels, intraperitoneal glucose tolerance, and gene expression were evaluated in mice treated with BSA or IL-25. Ob/ob mice fed a normal control diet were also treated with BSA or IL-25 and body weight and blood glucose levels were measured. Transepithelial electrical resistance and sodium-linked glucose absorption were determined in muscle-free small intestinal tissue and glucose absorption assessed in vitro in intestinal epithelial and skeletal muscle cell lines.
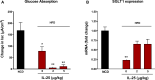
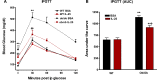
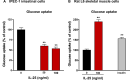
Results: Administration of IL-25 to HFD fed mice reversed glucose intolerance, an effect mediated in part by reduction in SGLT-1 activity and Glut2 expression. Importantly, the improved glucose tolerance in HFD mice treated with IL-25 was maintained for several weeks post-treatment indicating long-term changes in glucose metabolism in obese mice. Glucose intolerance was also reversed by IL-25 treatment in genetically obese ob/ob mice without inducing weight loss. In vitro studies demonstrated that glucose absorption was inhibited by IL-25 treatment in the epithelial IPEC-1 cells but increased glucose absorption in the L6 skeletal muscle cells. This supports a direct cell-specific effect of IL-25 on glucose metabolism.
Conclusion: These results suggest that the IL-25 pathway may be a useful target for the treatment of metabolic syndrome.
Keywords: glucose; metabolic syndrome; ob/ob; rodent.
© 2021 Smith et al.
Conflict of interest statement
This work was prepared while Aiping Zhao was employed at University of Maryland School of Medicine and his current address is Center for Scientific Review, National Institutes of Health, Bethesda, MD, USA. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government. Dr Aiping Zhao reports a patent US9724392B2 issued to University of Maryland Baltimore. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
High-fat diet induces leptin resistance in leptin-deficient mice.J Neuroendocrinol. 2014 Feb;26(2):58-67. doi: 10.1111/jne.12131. J Neuroendocrinol. 2014. PMID: 24382295
-
Altered Interleukin-10 Signaling in Skeletal Muscle Regulates Obesity-Mediated Inflammation and Insulin Resistance.Mol Cell Biol. 2016 Nov 14;36(23):2956-2966. doi: 10.1128/MCB.00181-16. Print 2016 Dec 1. Mol Cell Biol. 2016. PMID: 27644327 Free PMC article.
-
SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice.EBioMedicine. 2017 Jun;20:137-149. doi: 10.1016/j.ebiom.2017.05.028. Epub 2017 May 26. EBioMedicine. 2017. PMID: 28579299 Free PMC article.
-
The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties.Pharmacol Res. 2019 Dec;150:104487. doi: 10.1016/j.phrs.2019.104487. Epub 2019 Oct 11. Pharmacol Res. 2019. PMID: 31610229
-
Pancreastatin inhibitor PSTi8 protects the obesity associated skeletal muscle insulin resistance in diet induced streptozotocin-treated diabetic mice.Eur J Pharmacol. 2020 Aug 15;881:173204. doi: 10.1016/j.ejphar.2020.173204. Epub 2020 May 19. Eur J Pharmacol. 2020. PMID: 32439261
Cited by
-
Possible connection between intestinal tuft cells, ILC2s and obesity.Front Immunol. 2024 Jan 12;14:1266667. doi: 10.3389/fimmu.2023.1266667. eCollection 2023. Front Immunol. 2024. PMID: 38283340 Free PMC article. Review.
-
Discovery and multi-parametric optimization of a high-affinity antibody against interleukin-25 with neutralizing activity in a mouse model of skin inflammation.Antib Ther. 2022 Sep 29;5(4):258-267. doi: 10.1093/abt/tbac022. eCollection 2022 Oct. Antib Ther. 2022. PMID: 36299415 Free PMC article.
-
Obesity-Mediated Immune Modulation: One Step Forward, (Th)2 Steps Back.Front Immunol. 2022 Jun 30;13:932893. doi: 10.3389/fimmu.2022.932893. eCollection 2022. Front Immunol. 2022. PMID: 35844529 Free PMC article. Review.
References
-
- Katsuki A, Sumida Y, Murashima S, et al. Serum levels of tumor necrosis factor-α are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83(3):859–862. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

