Organic Anion Transporting Polypeptide 2B1 in Human Fetal Membranes: A Novel Gatekeeper for Drug Transport During Pregnancy?
- PMID: 34987396
- PMCID: PMC8721670
- DOI: 10.3389/fphar.2021.771818
Organic Anion Transporting Polypeptide 2B1 in Human Fetal Membranes: A Novel Gatekeeper for Drug Transport During Pregnancy?
Abstract
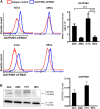
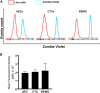
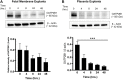
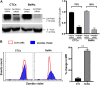
Current intervention strategies have not been successful in reducing the risks of adverse pregnancy complications nor maternal and fetal morbidities associated with pregnancy complications. Improving pregnancy and neonatal outcomes requires a better understanding of drug transport mechanisms at the feto-maternal interfaces, specifically the placenta and fetal membrane (FM). The role of several solute carrier uptake transporter proteins (TPs), such as the organic anion transporting polypeptide 2B1 (OATP2B1) in transporting drug across the placenta, is well-established. However, the mechanistic role of FMs in this drug transport has not yet been elucidated. We hypothesize that human FMs express OATP2B1 and functions as an alternate gatekeeper for drug transport at the feto-maternal interface. We determined the expression of OATP2B1 in term, not-in-labor, FM tissues and human FM cells [amnion epithelial cell (AEC), chorion trophoblast cell (CTC), and mesenchymal cells] using western blot analyses and their localization using immunohistochemistry. Changes in OATP2B1 expression was determined for up to 48 h after stimulation with cigarette smoke extract (CSE), an inducer of oxidative stress. The functional role of OATP2B1 was determined by flow cytometry using a zombie violet dye substrate assay. After OATP2B1 gene silencing, its functional relevance in drug transport through the feto-maternal interface was tested using a recently developed feto-maternal interface organ-on-a-chip (OOC) system that contained both FM and maternal decidual cells. Propagation of a drug (Rosuvastatin, that can be transported by OATP2B1) within the feto-maternal interface OOC system was determined by mass spectrometry. FMs express OATP2B1 in the CTC and AEC layers. In FM explants, OATP2B1 expression was not impacted by oxidative stress. Uptake of the zombie violet dye within AECs and CTCs showed OATP2B1 is functionally active. Silencing OATP2B1 in CTCs reduced Rosuvastatin propagation from the decidua to the fetal AEC layer within the feto-maternal interface-OOC model. Our data suggest that TPs in FMs may function as a drug transport system at the feto-maternal interface, a function that was previously thought to be performed exclusively by the placenta. This new knowledge will help improve drug delivery testing during pregnancy and contribute to designing drug delivery strategies to treat adverse pregnancy outcomes.
Keywords: drug transport; human fetal membranes; organ-on-a-chip; pregnancy; transporter protein.
Copyright © 2021 Ganguly, Kammala, Benson, Richardson, Han and Menon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Fetal Membranes Contribute to Drug Transport across the Feto-Maternal Interface Utilizing the Breast Cancer Resistance Protein (BCRP).Life (Basel). 2022 Jan 23;12(2):166. doi: 10.3390/life12020166. Life (Basel). 2022. PMID: 35207454 Free PMC article.
-
Testing of drugs using human feto-maternal interface organ-on-chips provide insights into pharmacokinetics and efficacy.Lab Chip. 2022 Nov 22;22(23):4574-4592. doi: 10.1039/d2lc00691j. Lab Chip. 2022. PMID: 36322152 Free PMC article.
-
Feto-Maternal Interface Organ-on-Chip: A New Technology to Study Ascending Infection.Methods Mol Biol. 2024;2781:105-117. doi: 10.1007/978-1-0716-3746-3_10. Methods Mol Biol. 2024. PMID: 38502447
-
Organ-On-Chip Technology: The Future of Feto-Maternal Interface Research?Front Physiol. 2020 Jun 30;11:715. doi: 10.3389/fphys.2020.00715. eCollection 2020. Front Physiol. 2020. PMID: 32695021 Free PMC article. Review.
-
Stimulatory effect on the transport mediated by organic anion transporting polypeptide 2B1.Asian J Pharm Sci. 2020 Mar;15(2):181-191. doi: 10.1016/j.ajps.2019.10.004. Epub 2019 Nov 13. Asian J Pharm Sci. 2020. PMID: 32373198 Free PMC article. Review.
Cited by
-
Microfluidic chips in female reproduction: a systematic review of status, advances, and challenges.Theranostics. 2024 Jul 15;14(11):4352-4374. doi: 10.7150/thno.97301. eCollection 2024. Theranostics. 2024. PMID: 39113805 Free PMC article. Review.
-
The Role of Fetal Membranes during Gestation, at Term, and Preterm Labor.Placenta Reprod Med. 2023 Jan 31;2:4. doi: 10.54844/prm.2022.0296. Epub 2023 Mar 20. Placenta Reprod Med. 2023. PMID: 38304894 Free PMC article.
-
Transporter Regulation in Critical Protective Barriers: Focus on Brain and Placenta.Pharmaceutics. 2022 Jun 29;14(7):1376. doi: 10.3390/pharmaceutics14071376. Pharmaceutics. 2022. PMID: 35890272 Free PMC article. Review.
-
Review on new approach methods to gain insight into the feto-maternal interface physiology.Front Med (Lausanne). 2023 Nov 30;10:1304002. doi: 10.3389/fmed.2023.1304002. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38098843 Free PMC article. Review.
-
Acute Histological Chorioamnionitis and Birth Weight in Pregnancies With Preterm Prelabor Rupture of Membranes: A Retrospective Cohort Study.Front Pharmacol. 2022 Mar 4;13:861785. doi: 10.3389/fphar.2022.861785. eCollection 2022. Front Pharmacol. 2022. PMID: 35308217 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

