Pocket delipidation induced by membrane tension or modification leads to a structurally analogous mechanosensitive channel state
- PMID: 34986323
- PMCID: PMC9033278
- DOI: 10.1016/j.str.2021.12.004
Pocket delipidation induced by membrane tension or modification leads to a structurally analogous mechanosensitive channel state
Abstract
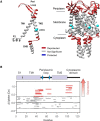
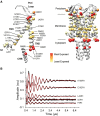
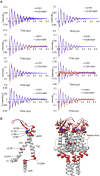
The mechanosensitive ion channel of large conductance MscL gates in response to membrane tension changes. Lipid removal from transmembrane pockets leads to a concerted structural and functional MscL response, but it remains unknown whether there is a correlation between the tension-mediated state and the state derived by pocket delipidation in the absence of tension. Here, we combined pulsed electron paramagnetic resonance spectroscopy and hydrogen-deuterium exchange mass spectrometry, coupled with molecular dynamics simulations under membrane tension, to investigate the structural changes associated with the distinctively derived states. Whether it is tension- or modification-mediated pocket delipidation, we find that MscL samples a similar expanded subconducting state. This is the final step of the delipidation pathway, but only an intermediate stop on the tension-mediated path, with additional tension triggering further channel opening. Our findings hint at synergistic modes of regulation by lipid molecules in membrane tension-activated mechanosensitive channels.
Keywords: EPR spectroscopy; ESSEM; HDX; MD; MscL; MscS; force-from-lipid; lipids; mass spectrometry; mechanosensitive channels.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

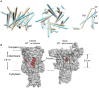


Similar articles
-
The gating mechanism of the bacterial mechanosensitive channel MscL revealed by molecular dynamics simulations: from tension sensing to channel opening.Channels (Austin). 2012 Jul-Aug;6(4):317-31. doi: 10.4161/chan.21895. Channels (Austin). 2012. PMID: 23146938 Free PMC article.
-
Visualization of the mechanosensitive ion channel MscS under membrane tension.Nature. 2021 Feb;590(7846):509-514. doi: 10.1038/s41586-021-03196-w. Epub 2021 Feb 10. Nature. 2021. PMID: 33568813
-
The activation mode of the mechanosensitive ion channel, MscL, by lysophosphatidylcholine differs from tension-induced gating.FASEB J. 2014 Oct;28(10):4292-302. doi: 10.1096/fj.14-251579. Epub 2014 Jun 23. FASEB J. 2014. PMID: 24958207 Free PMC article.
-
How do mechanosensitive channels sense membrane tension?Biochem Soc Trans. 2016 Aug 15;44(4):1019-25. doi: 10.1042/BST20160018. Biochem Soc Trans. 2016. PMID: 27528747 Review.
-
MscL: channeling membrane tension.Pflugers Arch. 2015 Jan;467(1):15-25. doi: 10.1007/s00424-014-1535-x. Epub 2014 May 27. Pflugers Arch. 2015. PMID: 24859800 Free PMC article. Review.
Cited by
-
Generating Ensembles of Dynamic Misfolding Proteins.Front Neurosci. 2022 Mar 31;16:881534. doi: 10.3389/fnins.2022.881534. eCollection 2022. Front Neurosci. 2022. PMID: 35431773 Free PMC article. Review.
-
Ligand binding and conformational dynamics of the E. coli nicotinamide nucleotide transhydrogenase revealed by hydrogen/deuterium exchange mass spectrometry.Comput Struct Biotechnol J. 2022 Sep 26;20:5430-5439. doi: 10.1016/j.csbj.2022.09.036. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36212541 Free PMC article.
-
EPR Spectroscopy Provides New Insights into Complex Biological Reaction Mechanisms.J Phys Chem B. 2022 Oct 6;126(39):7486-7494. doi: 10.1021/acs.jpcb.2c05235. Epub 2022 Sep 22. J Phys Chem B. 2022. PMID: 36137278 Free PMC article. Review.
-
Genetic and cellular characterization of MscS-like putative channels in the filamentous fungus Aspergillus nidulans.Channels (Austin). 2022 Dec;16(1):148-158. doi: 10.1080/19336950.2022.2098661. Channels (Austin). 2022. PMID: 35941834 Free PMC article.
-
Mechanosensitive channel MscL gating transitions coupling with constriction point shift.Protein Sci. 2024 Apr;33(4):e4965. doi: 10.1002/pro.4965. Protein Sci. 2024. PMID: 38501596
References
-
- Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindah E., Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25.
Publication types
MeSH terms
Substances
Grants and funding
- BB/M012573/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 219999/Z/19/Z/WT_/Wellcome Trust/United Kingdom
- 220628/Z/20/Z/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BB/S018069/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

